|
 |
- Search
| Neurointervention > Volume 16(1); 2021 > Article |
|
Abstract
Purpose
While previous studies have suggested that preoperative embolization of hypervascular spinal metastases may alleviate intraoperative blood loss and improve resectability, trends and driving factors for choosing this approach have not been extensively explored. Therefore, we evaluated the trends and assessed the factors associated with preoperative embolization utilization for spinal metastatic tumors using a national inpatient database.
Materials and Methods
The National Inpatient Sample database of the Healthcare Cost and Utilization Project was queried for patients undergoing surgical resection for spinal metastasis between January 1, 2005 and December 31, 2017. Patients undergoing preoperative embolization were identified; trends in the utilization of preoperative embolization were analyzed using the Cochran-Armitage test. Multivariable regression was conducted to assess factors associated with higher preoperative embolization utilization.
Results
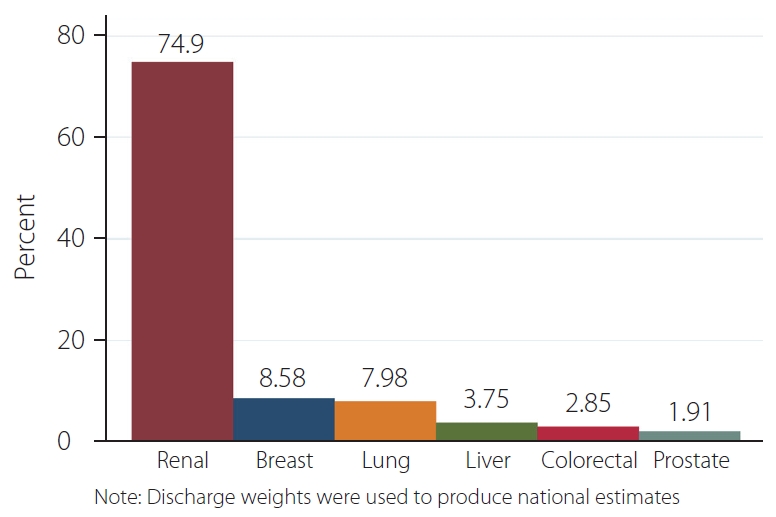
A total of 11,508 patients with spinal metastasis were identified; 105 (0.91%) underwent preoperative embolization. Of those 105 patients, 79 (75.24%) patients had a primary renal cancer, as compared to 1,732 (15.19%) of those who did not undergo preoperative embolization (P<0.001). The majority of patients in the non-preoperative embolization cohort had a primary lung tumor (n=3,562, 31.24%). Additionally, patient comorbidities were similar among the 2 groups (P>0.05). Trends in preoperative embolization indicated an increase of 0.16% (standard error: 0.024%, P<0.001) in utilization per year.
Conclusion
Utilization of preoperative embolization for spinal metastasis is increasing yearly, especially for patients with renal cancer, suggesting that surgeons may increasingly consider embolization before surgical resection for hypervascular tumors. Additionally, the literature has shown the intraoperative and postoperative benefits of this procedure.
Metastases to the spine may be due to primary tumors that are highly vascular, most commonly renal cell carcinoma [1]. While surgical resection is the mainstay for treatment of spinal metastasis, complications, such as excessive hemorrhage, can arise from surgical treatment of hypervascular metastases. Since the first embolization of bony metastasis recorded in the literature by Djindjian et al. [2] in 1973, preoperative embolization (PE) has become an established adjunct to improve outcomes and safety after resection of spinal metastases [3,4]. In a randomized controlled trial conducted by Clausen et al. [4], adjunct preoperative embolization of hypervascular spinal metastases resulted in decreased intraoperative blood loss from 645 mL to 902 mL during surgical resection. However, local practices in the utilization of preoperative embolization are quite variable, and overall trends of its usage in practice have not been assessed. Herein, we performed a trends analysis of preoperative embolization for spinal metastatic tumors using a national inpatient database.
The National Inpatient Sample (NIS) database of the Healthcare Cost and Utilization Project was queried for patients between January 1, 2005 and December 31, 2017. Every year, the NIS samples upwards of 5 million hospitalized patients, roughly 20% of all discharges annually. Largely developed for healthcare cost and utilization, the NIS is the largest public national database that represents all payers and is endorsed by the Agency for Healthcare Research and Quality [5]. No Institutional Review Board approval was required as the data was de-identified and collected from a national database.
Adults diagnosed with metastasis to the central nervous system, meninges, or bone between 2012 and 2017 were identified using International Classification of Diseases (ICD) 9th and 10th revision codes. Those with additional ICD-9 and ICD-10 procedure codes for spinal procedures were identified as patients with spinal metastasis; this method was previously demonstrated by Malik et al. [6], Kelly et al. [7], and Patil et al. [8]. Additionally, primary lung, breast, prostate, renal, liver, and colorectal tumors were identified using corresponding ICD-9 and ICD-10 diagnosis codes. Patients with unknown or multiple primary tumors were excluded from the cohort.
Total embolization of vessels was identified using ICD-9 and ICD-10 procedure codes. These codes were previously used in Brandel et al. [9], and Sideman and Zwolak [10]. Codes used in this study can be seen in Supplementary Tables 1 and 2.
To identify preoperative embolization cases, the variable “PRDAYn” was used to identify the day embolization was done after admission. This variable identifies the day in which a “PRn” (variable containing ICD procedure code) was done. Any patient with a PRDAY for embolization exceeding the day for the spinal procedure was identified with a postoperative embolization and was excluded from this study.
Discharge weights were used in this analysis to derive national estimates. These discharge weights are based on randomly selecting 20% of annual discharges. Selected discharges are then stratified by hospital characteristics. The weight is then derived by dividing the total number of discharges in that stratum by the number of discharges in that same stratum recorded in NIS [11].
Statistical analysis was performed using STATA 15 (Stata-Corp 2017, STATA Statistical Software: Release 15; StataCorp LP, College Station, TX, USA). P-values <0.05 were considered statistically significant. Continuous variables were presented as mean and standard deviation (SD), while categorical variables were presented as numbers and their corresponding percentages. The Student t-test was used to compare continuous variables between those who underwent preoperative embolization and those with surgery alone. Chi-square analysis was used to compare categorical variables between the 2 groups. Continuous variables were compared using a 2-sample t-test. STATA package “ptrend” was used to assess slope and significance in the rate of preoperative embolization between 2012–2017; “ptrend” conducts a Cochran-Armitage test for the trend of proportions over time [12]. Additionally, a trend subanalysis of primary renal tumors was conducted. Multivariable regression analysis was conducted to compare the patient profiles between the 2 groups; those results were represented by odds ratios (ORs) with 95% confidence intervals (CIs). Descriptive results were displayed as mean±SD or number (%) depending on the type of variable, continuous or categorical, respectively.
A total of 11,508 patients with spinal metastasis were identified; 105 (0.91%) underwent preoperative embolization. Of these 105 patients, 79 (75.24%) patients had a primary renal cancer, as compared to 1,732 (15.19%) of those who did not undergo preoperative embolization (P<0.001). The majority of patients in the non-PE cohort had a primary lung tumor (n=3,562, 31.24%). The average age between preoperative embolization and non-PE groups was 60.3±10.6 and 62.5±11.4, respectively. Additional demographic characteristics can be seen in Table 1. A bar graph of the distribution of primary tumors in the preoperative embolization group representing national estimates can be seen in Fig. 1.
Patients with primary renal tumors were more likely to undergo preoperative embolization compared to primary colorectal (OR: 0.08; 95% CI: 0.04–0.18; P<0.001), liver (OR: 0.08; 95% CI: 0.03–0.26; P<0.001), breast (OR: 0.19; 95% CI: 0.07–0.53; P=0.002), prostate (OR: 0.04; 95% CI: 0.02–0.10; P<0.001), and lung tumors (OR: 0.02; 95% CI: 0.004–0.072; P<0.001). Twenty-nine out of the 31 Elixhauser comorbidities were found to be similar between the 2 groups; patients with psychoses (OR: 4.45; 95% CI: 1.23–16.1; P=0.023) or cardiac arrhythmia (OR: 1.80; 95% CI: 1.09–2.96; P=0.022) were more likely to undergo preoperative embolization. Those with renal failure (OR: 0.79; 95% CI: 0.42–1.51; P=0.482) were found to be similar between the 2 groups. Admission in more recent years in our cohort had higher odds of undergoing preoperative embolization (OR: 1.26; 95% CI: 1.18–1.36; P<0.001). Patients who identify as Black or Hispanic had similar odds of undergoing preoperative embolization to those who identify as White (OR: 0.96; 95% CI: 0.42–2.18; P=0.925; OR: 1.10; 95% CI: 0.51–2.36; P=0.807, respectively). Those who identify as Asian, Pacific Islander, Native American, or Other/Combined Race were more likely to undergo preoperative embolization (OR: 1.97; 95% CI: 1.14–3.40; P=0.016). Additional results from the regression can be seen in Table 2.
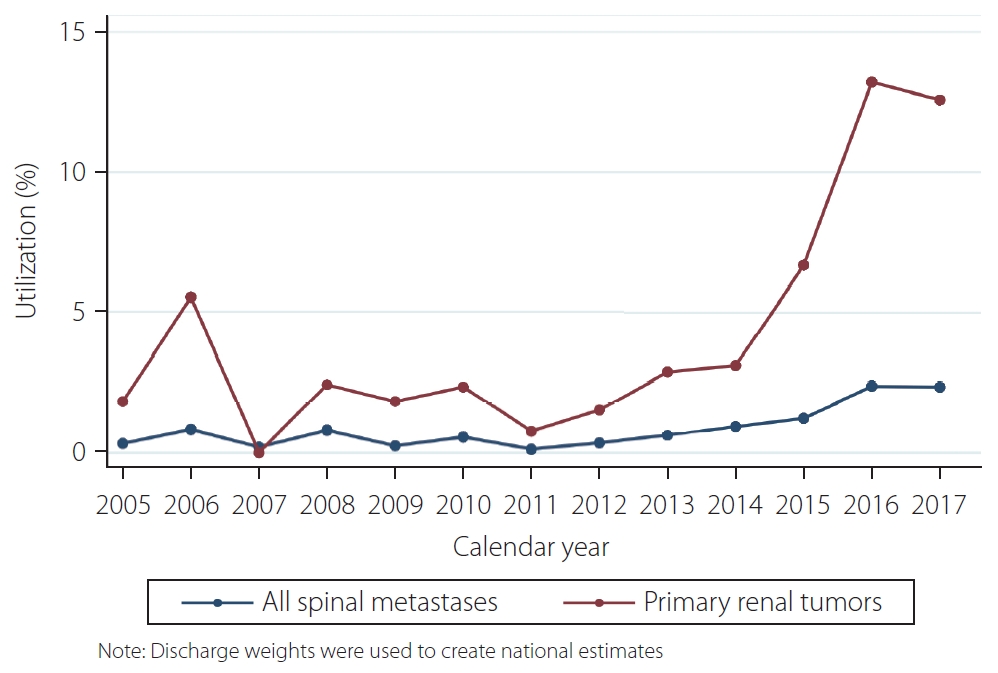
The Cochran-Armitage test indicated an average increase in utilization of 0.16% (standard error: 0.024%) per year (P<0.001). In 2005, utilization was 0.3%, compared to 2.3% in 2017. Subanalysis for trends of preoperative embolization for primary renal tumors indicated an average increase in utilization of 0.83% (standard error: 0.13%) per year (P<0.001). A line graph representing these trends using discharge weights to derive national estimates is shown in Fig. 2.
In an analysis of hospitalizations from the National Inpatient Sample, we observed an upward trend in utilization of preoperative embolization for spine tumors on a yearly basis between 2005 and 2017, with an average increase of 0.16% per year (P<0.001). This is also shown in the multivariable regression analysis, indicating that admissions in more recent years were more likely to undergo preoperative embolization (P<0.001). As expected, the most common primary cancer in patients undergoing preoperative embolization was renal cell cancer; and when only patients with renal cell primary cancer were considered, we found an average increase of 0.83%/year in the utilization of preoperative embolization. Additionally, patients who had cardiac arrhythmia (OR: 1.80; P=0.022) or psychoses (OR: 4.45; P=0.023) were more likely to undergo preoperative embolization. Patients who identify as Black or Hispanic had similar odds of undergoing preoperative embolization compared to those who identify as white (OR: 0.96; OR: 1.10, respectively). Those who identify as Other had higher odds of undergoing preoperative embolization compared to those who identify as White (OR: 1.97; 95% CI: 1.14–3.40; P=0.016). This may be attributable to the discrepancy in the sample sizes (17.5% in the preoperative embolization group vs. 10.4% in the non-preoperative embolization group).
In a meta-analysis of 6 studies, Luksanapruksa et al. [3] reported a significant reduction of intraoperative blood loss in patients undergoing preoperative embolization when compared to patients undergoing surgical resection of spinal metastases without preoperative embolization. With a “leave-one-out” analysis (the study with the smallest sample size), the mean difference in intraoperative blood loss was 1,226 mL less for the preoperative embolization group. The complication rate for preoperative embolization was acceptably low, and only 1 of the 6 studies considered in the aforementioned metanalysis reported complications related to embolization (3 minor: atrial fibrillation in 2 patients and a pleural exudate in another; and 1 major: common femoral artery thrombosis) [3].
While early reports on preoperative embolization of spinal metastases date around 1993, the majority were published after 2010 [3,13,14]. This trend in the literature parallels the trend identified in our study of increased utilization of preoperative embolization for spinal metastases. Several factors are responsible for this observation. The primary driver was increased awareness of this treatment option, instead of surgical resection previously. Second, technological advances in endovascular techniques, such as novel embolic agents, microcatheters, and more refined imaging, have made preoperative embolization a safer and more effective procedure over the years [15]. Additionally a deeper understanding of variant spinal vascular anatomy and anatomical contraindications, such as adjacent radiculomedullary arteries, have decreased the likelihood of complications [15].
There were limitations to this study. Regarding data collection, the NIS only contains hospitalizations, so preoperative embolizations performed in the outpatient setting were not included. However, since the database is nationally representative and discharges were weighted, it is suitable for the evaluation of trends of inpatient procedures. Second, since no ICD codes for spinal metastases exist, we used previously published studies’ methods of isolating and analyzing the same population and screened these codes carefully [6-8]. Third, we included years 2005–2017, which requires the use of both ICD-9 and ICD-10 codes. Fourth, intraoperative blood loss, a possible indicator of the efficacy of the preoperative embolization was unable to be measured in our analyses due to lack of coding for this metric. Nevertheless, our study shows trends in the increased use of preoperative embolization for spinal metastases, especially in patients with renal cell primary cancer.
Utilization of preoperative embolization for spinal metastasis is increasing yearly, especially for patients with primary renal cancer, suggesting that surgeons are increasingly embracing embolization prior to surgical resection for hypervascular tumors. Pre-operative embolization has a negligible role in the majority of less vascular metastases.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.5469/neuroint.2020.00381.
Notes
Ethics Statement
No IRB approval was required as the data was de-identified and collected from a national database.
Author Contribution
Concept and design: WW, AYA, and YUY. Analysis and interpretation: WW and AYA. Data collection: WW and YUY. Writing the article: WW and AYA. Critical revision of the article: WB, DFK, and GL. Final approval of the article: MB. Statistical analysis: WW. Overall responsibility: MB.
Table 1.
Patient characteristics
Table 2.
Characteristics that are associated with patients undergoing preoperative embolization
REFERENCES
1. Prince EA, Ahn SH. Interventional management of vertebral body metastases. Semin Intervent Radiol 2013;30:278-281.



2. Djindjian R, Houdart R, Rey A. [Place of embolization in the investigation and therapy of cerebral and spinal malformations and vascular tumors. (Apropos of 50 cases)]. Ann Med Interne (Paris) 1973;124:365-375 French

3. Luksanapruksa P, Buchowski JM, Tongsai S, Singhatanadgige W, Jennings JW. Systematic review and meta-analysis of effectiveness of preoperative embolization in surgery for metastatic spine disease. J Neurointerv Surg 2018;10:596-601.


4. Clausen C, Dahl B, Frevert SC, Hansen LV, Nielsen MB, Lönn L. Preoperative embolization in surgical treatment of spinal metastases: single-blind, randomized controlled clinical trial of efficacy in decreasing intraoperative blood loss. J Vasc Interv Radiol 2015;26:402-412 e1


5. NIS overview [Internet] Rockville, Agency for Healthcare Research and Quality. [cited 2020 May 2]. Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp
6. Malik AT, Baek J, Alexander JH, Khan SN, Scharschmidt TJ. Orthopaedic vs. neurosurgery - does a surgeon’s specialty have an influence on 90-day complications following surgical intervention of spinal metastases? Clin Neurol Neurosurg 2020;192:105735


7. Kelly ML, Kshettry VR, Rosenbaum BP, Seicean A, Weil RJ. Effect of a randomized controlled trial on the surgical treatment of spinal metastasis, 2000 through 2010: a population-based cohort study. Cancer 2014;120:901-908.


8. Patil CG, Lad SP, Santarelli J, Boakye M. National inpatient complications and outcomes after surgery for spinal metastasis from 1993-2002. Cancer 2007;110:625-630.


9. Brandel MG, Rennert RC, Wali AR, Santiago-Dieppa DR, Steinberg JA, Ramos C, et al. Impact of preoperative endovascular embolization on immediate meningioma resection outcomes. Neurosurg Focus 2018;44:E6.

10. Sideman M, Zwolak R. Coding for vascular and endovascular surgery. Edited by Savarise M, Senkowski C Principles of coding and reimbursement for surgeons, : Cham, Springer; 2017,pp 297-310.
11. Trend weights for HCUP NIS data [Internet] Rockville, Agency for Healthcare Research and Quality. [cited 2020 Jul 30]. Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp
12. Armitage P, Berry G, Matthews JNS. Statistical methods in medical research, 4th ed. : Hoboken, John Wiley & Sons; 2008.
13. Awad AW, Almefty KK, Ducruet AF, Turner JD, Theodore N, McDougall CG, et al. The efficacy and risks of preoperative embolization of spinal tumors. J Neurointerv Surg 2016;8:859-864.