|
 |
- Search
| Neurointervention > Volume 16(3); 2021 > Article |
|
Abstract
Flow diverters have become a critical instrument for complex aneurysms treatment. However, limited data are currently available regarding short and long-term outcomes for the Silk flow diverter. The objective of the study is to determine neurological prognosis and mortality rates for the Silk flow diversion device used in intracranial aneurysms. A systematic review with meta-analysis was performed using databases. The following descriptors were used for the search: ŌĆ£SILKŌĆØ, ŌĆ£Flow DiverterŌĆØ, ŌĆ£MortalityŌĆØ, and ŌĆ£PrognosisŌĆØ. The following data were extracted: mortality, good functional outcome, Glasgow outcome scale, complete or near-complete occlusion rates, rate of retreatment, and complications (thromboembolic and hemorrhagic complications). A total of 14 studies were selected. Among the 14 studies, 13 were retrospective observational cohort studies and 1 was a prospective observational cohort study. The mortality rate was 2.84%. The clinical good outcomes rate was 93.3%. The poor outcome rate was 6.6%. The overall thromboembolic complication rate was 6.06% (95% confidence interval [CI] 0.00ŌĆō6.37, P=0.12, I2=3.13%). The total hemorrhagic complication rate was 1.62% (95% CI 0.00ŌĆō5.34, P=0.28, I2=1.56%). The complete aneurysm occlusion rate was 80.4% (95% CI 8.65ŌĆō9.38, P<0.0001, I2=9.09%). The Silk diverter device has a good safety and efficacy profile for treating intracranial aneurysms with high complete occlusion rates.
The Silk stent device is a stent made of woven nitinol stands with low porosity folded in a plastic sheath, characterized by its flexibility and self-expanding properties (with a 2ŌĆō5 mm diameter and 15ŌĆō40 mm length presentation to choose from) [1,2]. Its porosity is 45ŌĆō60%, and it has a 9 mm distal radiopaque tip. It is deployed by careful pressure on the retraction of the delivery line and microcatheter; an advantage of the device is that it can be re-sheathed, even if it has been deployed up to 90%. Also, the conveying system has an improved pusher profile to achieve the best compromise between flexibility and pushability (the ability to transfer force to the distal end from the proximal end of the catheter) [2,3]. Since the introduction of auxiliary stent implantation for the treatment of fusiform aneurysms in the 1990s, in the past decade, Silk stents have been used to maintain blood flow in arteries, excluding aneurysms sacs [1]. These instruments were known as the flow diverter (FD) devices, and they have become critical instruments; several kinds have been used to treat intracranial aneurysms (IAs) over recent years [1,4,5]. The first FD to obtain European Commission approval was the Silk flow diverter (Balt Extrusion, Montmorency, France) in 2008. Eventually, short-and mid-term findings have been reported [1,6,7]. FDs redirect blood flow from the aneurysm, prevent thrombosis development, promote neo-intimal growth along with the mesh, and reconstruct the parent artery [8]. The safety and efficacy of FD treatment for many complex IAs have been recorded in numerous studies [9ŌĆō11]. In the Briganti and collaborators [12] systematic review, more than half of small IAs (56%) were treated with FDs. Despite the vast number of studies on the effectiveness of FDs in treating aneurysms, numerous unexpected adverse effects have also been reported. In addition, there is a lack of research on the problems associated with this approach. Few studies have examined clinical and technological incidents in the use of FDs to treat IAs systematically. The Silk Flow Diverter is a self-expandable stent braided from 48 nitinol wires. The DIVERSION registry is a prospective multicenter study covering all consecutive FD patients in French participating centers between October 2012 and February 2014 [12]. IAs care remains a significant obstacle for modern medicine. In massive subarachnoid dissections and in using multiple clips for artery reconstruction in cases of dysplastic and giant aneurysms, surgical developments have been introduced [13]. However, the Silk FD has shown mounting evidence in the management of this disease; therefore, the primary goal of this study was to determine the effect of the Silk flow diverter device on neurological prognosis and mortality in intracranial aneurysms.
We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for the design of this study.
A search for RCT, not-RCT, prospective, and retrospective cohort studies was carried out in SCOPUS (until October 2020), Central Cochrane Registry of Controlled Trials (The Cochrane Library) (until October 2020), MEDLINE (Ovid) until October 2020, EMBASE (Ovid), and PubMed (https://pubmed.ncbi.nlm.nih.gov/) (until October 2020), in addition to the reference list of included studies and other relevant data in potentially eligible studies.
The following search was used: ŌĆ£SILKŌĆØ AND (ŌĆ£flow diverterŌĆØ OR ŌĆ£flow diversionŌĆØ) AND (ŌĆ£MortalityŌĆØ OR ŌĆ£DeathŌĆØ OR ŌĆ£deadŌĆØ) AND (ŌĆ£PrognosisŌĆØ OR ŌĆ£morbidityŌĆØ OR ŌĆ£ComplicationŌĆØ) NOT ŌĆ£AnimalsŌĆØ.
The studies to be included were screened separately using the following inclusion criteria:
ŌĆó Patients with intracranial aneurysms treated with the Silk device.
ŌĆó RCT, not-RCT, prospective, retrospective cohort studies, case series with more than 20 patients included assessing mortality, functional prognosis type modified Rankin scale (mRS), Glasgow outcome scale (GOS), or another.
The quality of included studies was determined using the NewcastleŌĆōOttawa Quality Assessment Scale; studies with a score of 7 were considered of high methodological quality. Those with scores in the range of 4 to 5 were considered of moderate quality.
The following data were extracted: mortality, good functional outcome defined as mRS 0 to 2 points, GOS 4 to 6 points, complete or near-complete occlusion (OŌĆÖKellyŌĆōMarotta CŌĆōD, >90% or another scale) at the end follow-up, rate of retreatment, thromboembolic complications (thrombosis, cerebral infarct, or embolism), and hemorrhagic complications (ruptured aneurysm, subarachnoid hemorrhage, intracerebral hemorrhage). The authors of the included studies were contacted in case of missing data. Doubts were clarified by consensus. Statistical analysis was performed using a pooled rate with the Mantel-Haenszel methodology for each variable. MEDCALC software version 19.2 (MedCalc Software Ltd., Ostend, Belgium) with a randomized effect analysis model was used. Heterogeneity was assessed by calculating chi-square (I2), with high heterogeneity of studies included in the analysis being above 60%.
After conducting a systematic search of the information following our strategy, 70 bibliographic citations were identified from PUBMED, SCOPUS, EMBASE, MEDLINE, and Central Cochrane Registry of Controlled Trials (The Cochrane Library). After removing duplicate records, 44 were considered, and among those, 34 were screened. After screening, 20 were excluded, and 14 were potentially eligible based on title or abstract, or both, and full texts were obtained (Fig. 1) [2,6,14ŌĆō25]. All prospective and retrospective observational cohort studies were included.
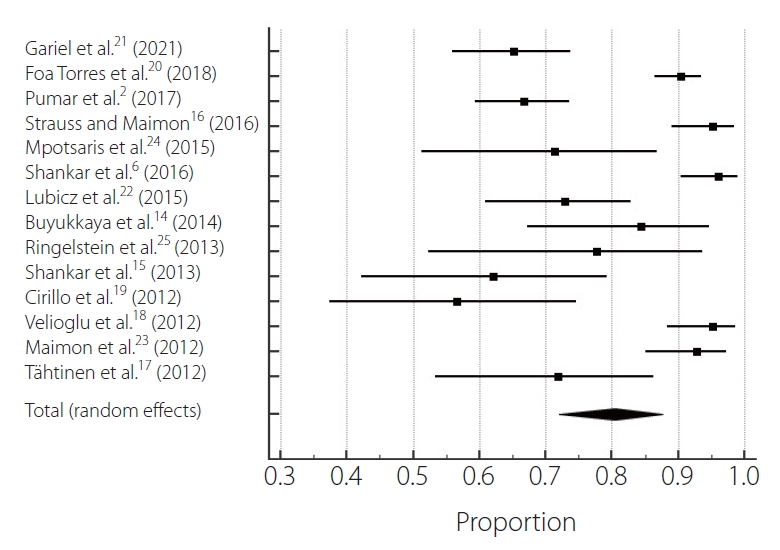
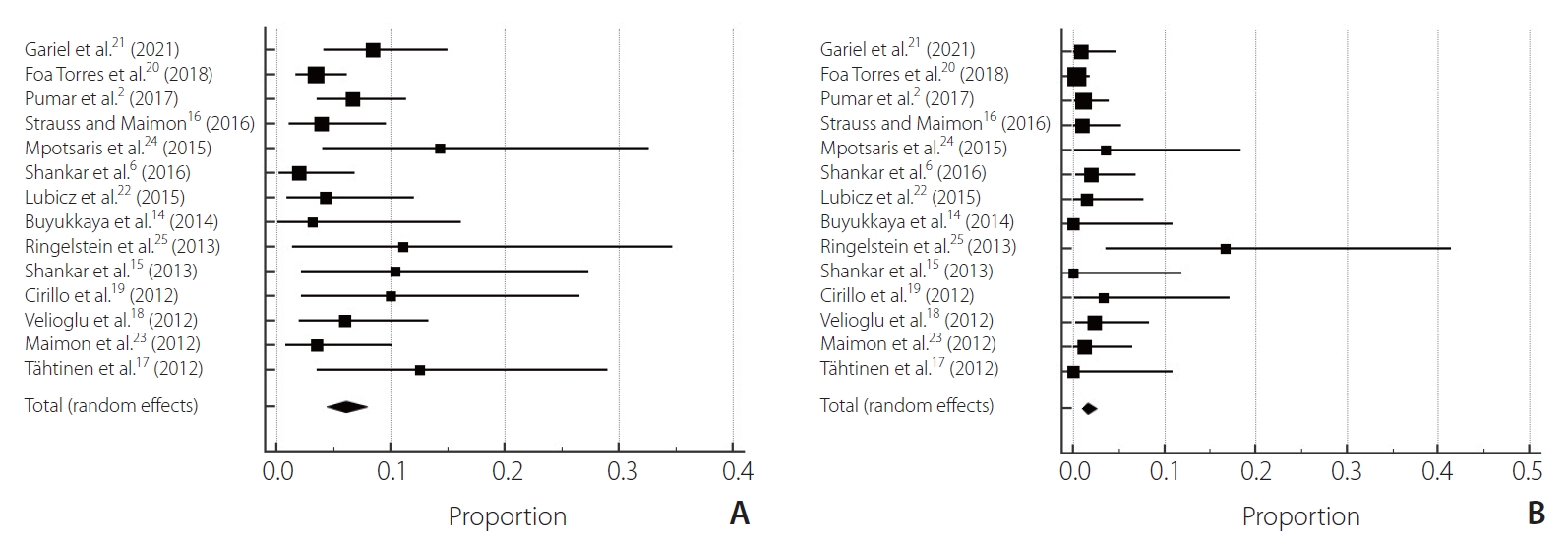
After selection, 14 studies were chosen [2,6,14ŌĆō25], 1 prospective observational cohort study and 13 retrospective observational cohort studies involving 1,021 patients treated for aneurysms [2,6,14ŌĆō20,22ŌĆō25]. The mortality rate was 2.84% (95% confidence interval [CI] 0.00ŌĆō2.75, P=0.83, I2=0%) (Table 1). The clinical good outcomes rate was 93.3% (95% CI 0.00ŌĆō6.23, P=0.15, I2=2.85%). The poor outcome rate was 6.6% (95% CI 0.00ŌĆō6.23, P=0.15, I2=2.85%) (Fig. 2). Regarding complication rates, the total thromboembolic complication rate was 6.06% (95% CI 0.00ŌĆō6.37, P=0.12, I2=3.13%). The total hemorrhagic complication rate was 1.62% (95% CI 0.00ŌĆō5.34, P=0.28, I2=1.56%) (Fig. 3). The complete aneurysm occlusion rate was 80.4% (95% CI 8.65ŌĆō9.38, P<0.0001, I2=9.09%) (Fig. 4).
Among the 14 included studies in the analysis, quality assessment was overall good. Three studies scored 7 in NewcastleŌĆōOttawa scale (NOS), 5 scored 6, and 6 scored 5 (Table 2). The funnel plot for all predictors showed different grades of asymmetry (Supplementary Figs. 1ŌĆō3).
Combining data from 14 trials, this systematic study and meta-analysis of 1,021 aneurysm patients provide representative data on therapy with the Silk flow diverter to assess clinical outcomes, prognosis, and mortality rates. We have shown that Silk flow diversion treatment is clinically feasible with high technical success rates and high complete occlusion rates and safe with good long-term neurological performance. It should be noted that the complication rates associated with the treatment of these aneurysms have not been negligible.
Our analysis indicated that the mortality rate was 2.84%. In the findings of the studies that were included, some heterogeneity was identified. There is a shortage of research relevant to this methodology on protection and morbidity, so the data added by this study can be helpful. The morbidity and mortality rates were reported in percentage ranges from 2.8 percent to 14.1 percent and 0 percent to 3.7 percent in several recent data studies, respectively, with a permanent morbidity rate of 1.3 percent to 6.3 percent [2,6,14ŌĆō25]. Our research has several merits compared to previously published papers. Since our research covers all the studies available in this important area, it thoroughly reviews all the evidence available in these techniques on adverse events. Due to the rapid growth of the field, we analyzed a larger number of studies. Therefore, we can provide the most definitive summary available at present.
In a prospective Japanese cohort of unruptured IAs, 89.6 percent of the observed 6,679 aneurysms were reported [26]. FDs were used to treat complex and fusiform IAs, rather than small IAs. However, the treatment of small and less complex IAs with FDs has been documented in some studies. Our study showed that the total aneurysm occlusion rate was 80.4 percent (P<0.0001). The aneurysm occlusion rate for the small aneurysm subgroup was 80 percent at 6 months in BrinjikjiŌĆÖs meta-analysis [27]. In a population comprising 1,451 patients with 1,654 treated aneurysms, Brinjikji and colleagues [27] found no impact of aneurysm size on aneurysm occlusion rates. At the 9-month follow-up, Arrese et al. [28] recorded a 76.2 percent aneurysm occlusion rate of 26. No other discrepancies were noticed except for the types of instruments used. In the meta-analysis of Arrese et al. [28], aneurysm occlusions had high heterogeneity in the fixed-effects model. Aneurysm occlusion is a standard criterion that is used to assess intracranial aneurysm therapeutic outcomes. Single-center and multicenter research has shown that the 6-month aneurysm occlusion rate is between 38% and 100% [29]. In those studies with long-term follow-ups, the occlusion rate extension increased with increased time [27ŌĆō29]. According to the meta-analyses by Arrese et al. [28], the occlusion of the aneurysm was 76.2% at a mean follow-up of 9 months.
The treatment of complex intracranial aneurysms with the Silk flow diverter is an effective therapeutic choice. The new Silk stent seems better, despite the delayed complications. In addition, at long-term follow-up, this endovascular approach obtained a high rate of sufficient and stable aneurysm occlusion. The recent implementation of intermediate access catheters also helps to promote endovascular procedures by providing substantial assistance to facilitate and enhance the delivery of stents. The high degree of effective therapy is consistent with previous series [17ŌĆō19,22,30]. A major concern regarding the use of FD stents is the rate of immediate and delayed complications. The good clinical outcome compares favorably with the recent meta-analysis by Murthy et al. [30]. Indeed, in larger intracranial aneurysms in which additional coiling is now recommended, the rate of delayed complications is significantly higher [30]. In previous studies, no recanalization or decrease in occlusion grade was found [22,31].
Our findings demonstrated an overall complication rate of 17%. After Silk flow diverter implantation, there are few reports on acute complications, as most studies report combined rates of acute, subacute, and delayed complications. Most of the complications in our experience were ischemic complications similar to those described by Berge et al. [32]. For Silk flow diverter stents, Berge et al. [32] also reported 7.8 percent transient in-stent stenosis and asymptomatic patients. Like other aneurysm patterning stents, FD stents can induce intracranial hemorrhage and intraluminal stenosis within the parent artery.
The limitations of the present study are a lack of data according to delayed rupture following the endovascular procedure. Our scope did not consider a subgroup analysis according to the follow-up period; also, any resulting differentiation of the Silk flow diverter is a limitation of the present study due to the inability to analyze correspondent data.
The Silk diverter device has a good safety and efficacy profile for treating intracranial aneurysms with high complete occlusion rates. With appropriate mortality and morbidity rates in complicated aneurysms, it is a safe care option that is unlikely to be handled with other techniques. There is, however, a chance of neurologic morbidity and ischemic incidents due to the procedure. It is essential to study the mechanism of delayed rupture after flow diversion to determine the required perioperative medication and optimal implantation method. Further studies are needed to provide reliable data on the techniqueŌĆÖs safety and delayed rupture after the procedure.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.5469/neuroint.2021.00234.
Notes
Ethics Statement
There was no need for an institutional ethics committee approval. Also, for this type of study formal consent is not required.
Author Contribution
Concept and design: TJ, SK, AA, and LRM. Analysis and interpretation: WAF. Data collection: WAF. Writing the article: WAF, EG, GAQ, TJ, and SK. Critical revision of the article: EG, GAQ, TJ, and AA. Final approval of the article: EG, GAQ, AA, and LRM. Statistical analysis: WAF. Overall responsibility: GAQ, AA, and LRM.
Fig.┬Ā1.
PRISMA flow chart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICO, patient, intervention, comparation, outcome.

Fig.┬Ā2.
Forest plot of clinical outcome: (A) mortality, (B) good neurological outcome, (C) poor neurological outcome.
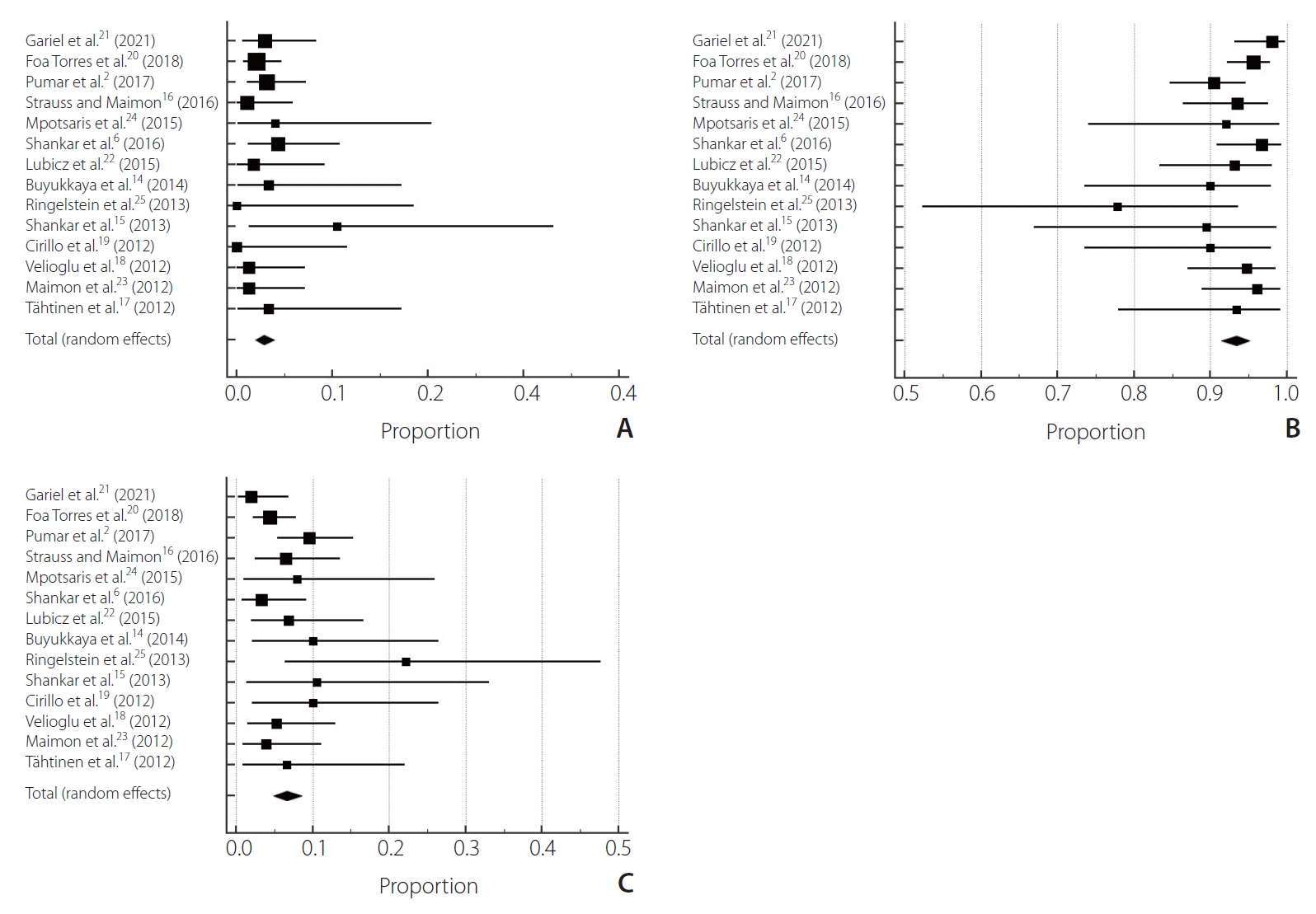
Fig.┬Ā3.
Forest plot pooled complications: (A) thromboembolic complications, (B) hemorrhagic complication.
Table┬Ā1.
Characteristics of included studies
| Study | Type | Number | Outcome assessed | Following length (mo) | NewcastleŌĆōOttawa scale score |
|---|---|---|---|---|---|
| Gariel et al. [21] (2021) | Prospective observational cohort study | Patients: 102 | Mortality prognosis: mRS | 12 | 7/7 |
| Treated aneurysm: 118 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Kamran scale | |||||
| Foa Torres et al. [20] (2018) | Retrospective observational cohort study | Patients: 246 | Mortality prognosis: GOS | 12 | 7/7 |
| Treated aneurysm: 293 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Pumar et al. [2] (2017) | Retrospective observational cohort study | Patients: 157 | Mortality prognosis: mRS | 6 | 5/7 |
| Treated aneurysm: 180 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Strauss and Maimon [16] (2016) | Retrospective observational cohort study | Patients: 92 | Mortality prognosis: mRS | 12 | 7/7 |
| Treated aneurysm: 103 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Mpotsaris et al. [24] (2015) | Retrospective observational cohort study | Patients: 25 | Mortality prognosis: mRS | 6 | 6/7 |
| Treated aneurysm: 28 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Shankar et al. [6] (2016) | Retrospective observational cohort study | Patients: 92 | Mortality prognosis: mRS | 24 | 5/7 |
| Treated aneurysm: 103 | Complications: New-onset Ischemia Raymond-Roy Oclussion scale | ||||
| Lubicz et al. [22] (2015) | Retrospective observational cohort study | Patients: 58 | Mortality prognosis: mRS | 12 | 5/7 |
| Treated aneurysm: 70 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: PAO scale | |||||
| Buyukkaya et al. [14] (2014) | Retrospective observational cohort study | Patients: 30 | Mortality prognosis: mRS | 6 | 6/7 |
| Treated aneurysm: 32 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Ringelstein et al. [25] (2013) | Retrospective observational cohort study | Patients: 18 | Mortality prognosis: mRS | 12 | 6/7 |
| Treated aneurysm: 18 | Complications: Hemorrhagic stroke | ||||
| Shankar et al. [15] (2013) | Retrospective observational cohort study | Patients: 19 | Mortality prognosis: mRS | 3 | 5/7 |
| Treated aneurysm: 29 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Cirillo et al. [19] (2012) | Retrospective observational cohort study | Patients: 30 | Mortality | 12 | 5/7 |
| Treated aneurysm: 30 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Velioglu et al. [18] (2012) | Retrospective observational cohort study | Patients: 76 | Mortality | 12 | 6/7 |
| Treated aneurysm: 84 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| Maimon et al. [23] (2012) | Retrospective observational cohort study | Patients: 76 | Mortality | 6 | 6/7 |
| Treated aneurysm: 84 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale | |||||
| T├żhtinen et al. [17] (2012) | Retrospective observational cohort study | Patients: 30 | Mortality prognosis: mRS | 6 | 5/7 |
| Treated aneurysm: 32 | Complications: stroke | ||||
| Intracranial bleeding occlusions aneurysm: Raymond-Roy Oclussion scale |
Table┬Ā2.
NewcastleŌĆōOttawa scale for quality assessment of studies included in this meta-analysis
| Study | Representativeness of sample | Size sample | Source of information | Demonstration that outcome was not present at study start | Confusion variable control | Assessment of outcome | Enough follow-up period | NewcastleŌĆōOttawa scale score |
|---|---|---|---|---|---|---|---|---|
| Gariel et al. [21] (2021) | * | * | * | * | * | * | * | 7/7 |
| Foa Torres et al. [20] (2018) | * | * | * | * | * | * | * | 7/7 |
| Pumar et al. [2] (2017) | * | * | * | * | * | 5/7 | ||
| Strauss and Maimon [16] (2016) | * | * | * | * | * | * | * | 7/7 |
| Mpotsaris et al. [24] (2015) | * | * | * | * | * | * | 6/7 | |
| Shankar et al. [6] (2016) | * | * | * | * | * | * | * | 5/7 |
| Lubicz et al. [22] (2015) | * | * | * | * | * | 5/7 | ||
| Buyukkaya et al. [14] (2014) | * | * | * | * | * | * | 6/7 | |
| Ringelstein et al. [25] (2013) | * | * | * | * | * | * | 6/7 | |
| Shankar et al. [15] (2013) | * | * | * | * | * | 5/7 | ||
| Cirillo et al. [19] (2012) | * | * | * | * | * | 5/7 | ||
| Velioglu et al. [18] (2012) | * | * | * | * | * | * | 6/7 | |
| Maimon et al. [23] (2012) | * | * | * | * | * | * | 6/7 | |
| T├żhtinen et al. [17] (2012) | * | * | * | * | * | 5/7 |
REFERENCES
1. Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One 2010;5:e12492.



2. Pumar JM, Banguero A, Cuellar H, Guimaraens L, Masso J, Miralbes S, et al. Treatment of intracranial aneurysms with the SILK embolization device in a multicenter study. A retrospective data analysis. Neurosurgery 2017;81:595-601.



3. Alderazi YJ, Shastri D, Kass-Hout T, Prestigiacomo CJ, Gandhi CD. Flow diverters for intracranial aneurysms. Stroke Res Treat 2014;2014:415653



4. DŌĆÖUrso PI, Lanzino G, Cloft HJ, Kallmes DF. Flow diversion for intracranial aneurysms: a review. Stroke 2011;42:2363-2368.


5. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007;38:2346-2352.


6. Shankar JJ, Tampieri D, Iancu D, Cortes M, Agid R, Krings T, et al. SILK flow diverter for complex intracranial aneurysms: a Canadian registry. J Neurointerv Surg 2016;8:273-278.


7. Wagner A, Cortsen M, Hauerberg J, Romner B, Wagner MP. Treatment of intracranial aneurysms. Reconstruction of the parent artery with flow-diverting (Silk) stent. Neuroradiology 2012;54:709-718.


8. Cant├│n G, Levy DI, Lasheras JC, Nelson PK. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005;103:891-902.


9. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858-868.


10. Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery 2005;56:E416discussion E416.


11. Piano M, Valvassori L, Quilici L, Pero G, Boccardi E. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurg 2013;118:408-416.


12. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 2015;28:365-375.



13. Derdeyn CP, Barr JD, Berenstein A, Connors JJ, Dion JE, Duckwiler GR, et al, Executive Committee of the American Society of Interventional and Therapeutic Neuroradiology; American Society of Neuroradiology. The International Subarachnoid Aneurysm Trial (ISAT): a position statement from the Executive Committee of the American Society of Interventional and Therapeutic Neuroradiology and the American Society of Neuroradiology. AJNR Am J Neuroradiol 2003;24:1404-1408.


14. Buyukkaya R, Kocaeli H, Yildirim N, Cebeci H, Erdogan C, Hakyemez B. Treatment of complex intracranial aneurysms using flow-diverting silk® stents. An analysis of 32 consecutive patients. Interv Neuroradiol 2014;20:729-735.



15. Shankar JJ, Vandorpe R, Pickett G, Maloney W. SILK flow diverter for treatment of intracranial aneurysms: initial experience and cost analysis. J Neurointerv Surg 2013;5 Suppl 3:iii11-iii15.


16. Strauss I, Maimon S. Silk flow diverter in the treatment of complex intracranial aneurysms: a single-center experience with 60 patients. Acta Neurochir (Wien) 2016;158:247-254.


17. T├żhtinen OI, Manninen HI, Vanninen RL, Sepp├żnen J, Niskakangas T, Rinne J, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery 2012;70:617-623 discussion 623-624


18. Velioglu M, Kizilkilic O, Selcuk H, Kocak B, Tureci E, Islak C, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology 2012;54:1355-1365.


19. Cirillo L, Leonardi M, DallŌĆÖolio M, Princiotta C, Stafa A, Simonetti L, et al. Complications in the treatment of intracranial aneurysms with silk stents: an analysis of 30 consecutive patients. Interv Neuroradiol 2012;18:413-425.



20. Foa Torres G, Roca F, Noguera A, Godes J, Petrocelli S, Aznar I, et al. Silk flow-diverter stent for the treatment of complex intracranial aneurysms: a one-year follow-up multicenter study. Interv Neuroradiol 2018;24:357-362.



21. Gariel F, Marnat G, Barreau X, Menegon P, Bourcier R, Pierot L, et al, DIVERSION investigators. Safety and efficacy of the Silk flow diverter: insight from the DIVERSION prospective cohort study. J Neuroradiol 2021;48:293-298.


22. Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F. Silk flow-diverter stent for the treatment of intracranial aneurysms: a series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol 2015;36:542-546.



23. Maimon S, Gonen L, Nossek E, Strauss I, Levite R, Ram Z. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 yearsŌĆÖ experience with 28 patients at a single center. Acta Neurochir (Wien) 2012;154:979-987.


24. Mpotsaris A, Skalej M, Beuing O, Eckert B, Behme D, Weber W. Long-term occlusion results with SILK flow diversion in 28 aneurysms: do recanalizations occur during follow-up? Interv Neuroradiol 2015;21:300-310.



25. Ringelstein A, Schlamann M, Goericke SL, M├Čnninghoff C, Sandalcioglu IE, El Hindy N, et al. [3-Year follow-up after endovascular aneurysm treatment with Silk┬« flow diverter]. Rofo 2013;185:328-332 German

26. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011;10:626-636.


27. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013;44:442-447.


28. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery 2013;73:193-199 discussion 199-200

29. Zhou G, Su M, Zhu YQ, Li MH. Efficacy of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. World Neurosurg 2016;85:252-262.


30. Murthy SB, Shah S, Shastri A, Venkatasubba Rao CP, Bershad EM, Suarez JI. The SILK flow diverter in the treatment of intracranial aneurysms. J Clin Neurosci 2014;21:203-206.


- TOOLS