 |
 |
- Search
| Neurointervention > Volume 17(2); 2022 > Article |
|
Abstract
The transradial approach (TRA) is an effective and safe alternative to transfemoral access for diagnostic neuroangiography and craniocervical interventions. While the technical aspects of supraclavicular intervention are well-described, there are little data on the TRA for thoracolumbar angiography and intervention. The authors describe the feasibility of the TRA for preoperative thoracic tumor embolization, emphasizing technique, device selection, navigation, and catheterization of thoracolumbar segmental arteries. This approach extends the benefits of TRA to spinal interventional neuroradiology.
Hypervascular tumors of the vertebral column present a considerable challenge for spine surgeons. Preoperative trans-arterial tumor embolization is an established tool for reducing operative blood loss and periprocedural morbidity in these patients [1,2]. This technique is well-established and has been widely practiced for over 30 years with relatively low complication rates [3,4]. Conventional transfemoral arterial access has been the standard approach for this procedure and benefits from tried-and-true techniques and an arsenal of catheters designed specifically for navigation of the descending aorta and its branches [5].
Recently, the transradial approach (TRA) for arterial access has emerged as a versatile approach for neuroendovascular surgery [6]. This approach has proved safe when appropriate techniques and precautions are employed [7]. Although the rapidly growing literature asserts non-inferiority benefits of the TRA, these reports are largely limited to cervical and intracranial interventions. To date, reports of the TRA for spinal intervention are limited [8-10]. These investigations do not address the technical aspects necessary for translation into routine clinical practice.
We present the feasibility of the TRA for spine tumor embolization, emphasizing indications, technique, and anatomic considerations.
A patient with metastatic cholangiocarcinoma was diagnosed with multiple spinal metastases, including a holo-vertebral lesion at T3 with extension to the epidural space, left neural foramen, and disruption of the posterior cortex. The patient was referred to surgical management, and preoperative embolization was requested. A review of abdominopelvic computed tomography (CT) showed extensive aortoiliac atherosclerotic disease but did not show definitive occlusion. The patient reported tenderness to palpation in the upper back but did not have frank myelopathy or radiculopathy. A review of systems and laboratory values were otherwise normal.
Initial vascular access was attempted using conventional landmark-guided, single-wall puncture of the right common femoral artery (CFA), but the guidewire (The 0.035 inch Bentson Guidewire; Cook Medical, Bloomington, IN, USA) could not be advanced beyond the external iliac artery. Contralateral CFA access was achieved with a similar technique, but the wire again encountered abnormal resistance. Given the known advanced aortoiliac bifurcation disease, we abandoned the transfemoral strategy for a TRA.
After confirming adequate palmar arch collaterals (Barbeau test), radial arteriotomy was performed using ultrasound guidance with a 21-gauge needle. A nitrex microwire was advanced, and a hydrophilic, 5 French Glidesheath Slender (Terumo Medical, Somerset, NJ, USA) was placed. A pre-treatment “cocktail” comprising 200 µg nitroglycerin, 2.5 mg verapamil, and 2,000 U heparin sulfate was slowly administered via the sheath. Using roadmap guidance, a Penumbra Sim 130 cm catheter (Penumbra Neurovascular, Alameda CA, USA) was advanced over a 0.035 inch Glidewire (Terumo Medical) and formed in the aortic arch following a single-injection anteroposterior aortogram, which confirmed chronic aortic occlusion with prominent collaterals reconstituting the femoral arteries.
Using this catheter, we proceeded to perform diagnostic angiography of the bilateral subclavian arteries, the right T4 and T5 segmental arteries, and finally, the left T3 segmental artery, which supplied T2 and T3 intercostal branches. A dense, hypervascular tumor blush was noted to arise from the left conjoined T2/T3 branch, and no spinal arteries were visualized (Fig. 1A). After selecting the ostium, the Simmons 2 catheter was withdrawn slightly to achieve a stable position proximally. Next, a Prowler Plus 0.021-inch microcatheter (Codman Neurovascular, Raynham, MA, USA) was advanced over a Transend EX Platinum microwire (Stryker Neurovascular, Fremont, CA, USA) through the diagnostic catheter. The microwire was then removed. Through the microcatheter, particle embolization was performed using a slurry of 100% Omnipaque 300 contrast and 150–250 micron polyvinyl alcohol particles. We then serially embolized tumor branches from a distal position until stasis of flow was observed; at each branch point, an additional embolic agent was administered to achieve complete stagnation of flow. A 2x5 mm pushable coil (Boston Scientific, Marlborough MA, USA) was then deployed in the radicular artery trunk. The post-embolization selective angiogram (Fig. 1B) showed excellent angiographic results with significant tumor blush reduction.
The patient recovered well from the endovascular procedure without complication from the access sites (bilateral CFA, right radial artery). Radial access closure was achieved with a radial compression band (RadAR; Semler Technologies, Milwaukie, OR, USA) applied for 2 hours, following sheath withdrawal with patent hemostasis and adequate pulse oximetry of the thumb. The following day, spinal surgery including T1-T10 posterior fusion, complete T3 corpectomy, and anterior fusion of T2-T4 proceeded without complication, with 2,000 mL estimated blood loss. The patient recovered quickly and was discharged on post-surgical day 2.
This report demonstrates the feasibility of the TRA as an alternate approach to therapeutic preoperative spinal tumor embolization. Our findings add to the growing literature supporting this technique in vascular access for neurointerventional procedures. The following is a point-by-point discussion of the technical considerations, both material and anatomic, which should be considered prior to engaging in the TRA for spinal procedures.
The access phase of the TRA for spinal procedures proceeds identically to our practice’s routine for cerebral angiography. The remainder of the procedure, however, requires several technical considerations, including arch anatomy, curvature of the descending aorta, segmental and lumbar anatomy, and age-related atherosclerosis. In most patients, the course from radial artery to descending aorta is relatively conventional in which a diagnostic catheter is advanced over a hydrophilic guidewire without significant technical difficulty. In our practice, this initial navigation is performed using angiographic roadmapping to identify variant anatomy (e.g., high brachial bifurcation, tortuous loop) [6]. Navigating the transition from innominate artery to aortic arch presents a variable challenge, but it was not difficult in this case. Theoretically, excess aortic stiffness and angulation can substantially increase torque on the catheter, rendering even to-and-fro movements cumbersome and resulting in longer procedure times [11]. Such anatomy, if known a priori, may require stiffer or larger diameter diagnostic catheters to avoid loss of kinetic energy translation at the arch. Therefore, the selection of diagnostic catheter is the first critical decision point that requires consideration of not only the aortic arch characteristics but also target anatomy (e.g., segmental, lumbar, and iliac arterial catheterization).
Haynes et al. [8] described a small series of intra-operative diagnostic angiography using the TRA, reporting success with Simmons 1 (T4, T10, T11) and Vertebral catheters (T8 and T10). The first depiction of the TRA for spinal embolization was presented by Orru et al. [10] in the case of a spinal dural arteriovenous fistula in 2019. These authors were able to target the lateral sacral artery to embolize a sacral dural arteriovenous fistula using a Sofia Plus 070 131 cm (MicroVention, Aliso Viejo CA, USA) catheter navigated directly over a shapeable glidewire. While an excellent strategy for iliac artery branch navigation, it is only a partial solution to the challenge of TRA spinal procedures. Comprehensive spinal angiography with the TRA must enable catheterization of the cervical, thoracic, and lumbar spine as well as pelvic vasculature [5]. Medullary arteries of the cervical spinal cord arise from the vertebral arteries, which are routinely catheterized in transradial cerebral angiography [6]. While the right vertebral artery is easier to catheterize from the right radial artery, the left vertebral artery may be catheterized most successfully with a Simmons II or Simmons III diagnostic catheter [12]. However, the segmental artery origins of the thoracic and lumbar spinal arteries present the most considerable challenge for TRA spinal intervention. These metameric vessels arise along the posterior or posterolateral aorta, with progressively less interdigitating space as the aorta descends to the lumbar arteries. Commonly, the lower lumbar artery origins are conjoined.
Several widely used catheters have been designed for accessing the segmental arteries from a transfemoral approach. The choice of catheter tip shape depends on the degree of caudal angulation of the segmental or lumbar artery origins with respect to the aorta. In most cases, the vessel origin angulation to the aorta becomes more caudal as the spine descends; however, this may be affected by an eccentric atherosclerotic plaque of the aorta. Noninvasive imaging of the spine, such as magnetic resonance or CT angiography, may aid in pre-procedural planning.
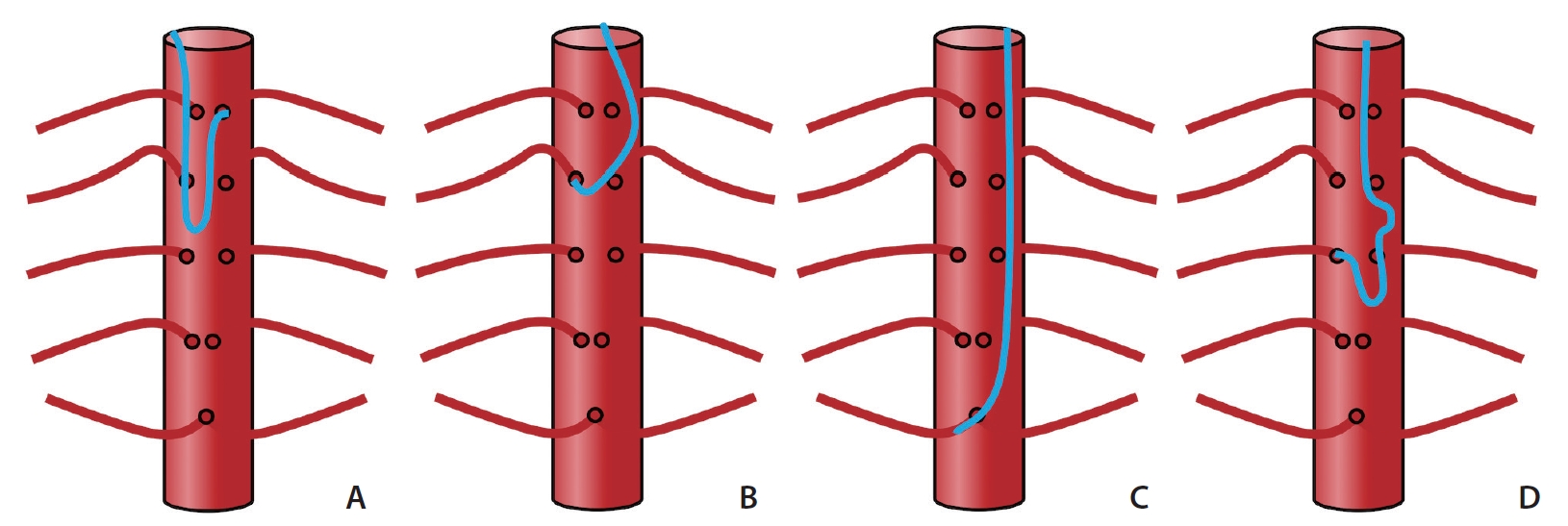
Compared with transfemoral access, the TRA inverts the angle and orientation of the catheter with respect to vessel origin. From the TRA, the catheter tip which is most cranially directed is the “Cobra” or “C2” shaped (Cordis, Fremont, CA, USA) catheter; and conversely, the most caudally oriented is the traditional angled vertebral catheter (Cook Medical). Theoretically, in the TRA, cranially-directed catheters are optimal in catheterizing spinal arteries in the upper thoracic whereas caudally-directed catheters are ideal for lower lumbar spinal arteries, due to congruency with the catheter tip and vessel angulation. Applying this principle, our group has had success using the 130 cm Penumbra Vert Catheter (Penumbra Neurovascular) for an L4 aneurysmal bone cyst embolization (Fig. 2). Additional catheters commonly used in spinal angiography include the Simmons 1, Simmons 2, and Mikaelson catheters (Angiodynamics, Latham, NY, USA), the latter of which has a more laterally oriented tip. As such, these catheters may be most appropriate for selecting mid-aorta segmental arteries. A schematic summary of catheter tip angles and their relative utility at different thoracolumbar levels is summarized in Fig. 3.
Besides catheter tip shape, another important consideration in TRA spinal angiography is catheter length. While standard catheter lengths are generally sufficient for thoracolumbar segmental arteries, TRA catheterization of the iliac arteries may prove to be more challenging, especially for taller patients. From this perspective, TRA catheterization of the iliac arteries is more dependent on catheter length than tip shape. As such, a catheter with a length greater than 100 cm or 125 cm is necessary and may require an exchange [8,10].
Once a catheter with adequate tip shape and length is positioned in the descending aorta, navigation from vessel to vessel using the TRA is performed with gentle “to-and-fro” advancement and withdrawal of the posteriorly-directed catheter until a segmental artery is selectively “hooked”. Depending on the tip shape, the catheter is then advanced (single-curve catheters) or pulled back (double-curve catheters) to seat the catheter into a stable position. This TRA technique to select and seat the catheter is the same as a transfemoral approach. Once the catheter is well-seated, the remainder of the embolization procedure proceeds identically to conventional transfemoral methods.
The TRA was essential in this case as transfemoral access was precluded by long-standing occlusion of the aortoiliac junction. The principal advantage of the TRA in this setting is the added flexibility when transfemoral access is technically infeasible. This is particularly important considering the typically advanced age and atherosclerotic risk factors prevalent in patients undergoing spinal neuroendovascular procedures [13]. Other advantages of the TRA in this scenario extend naturally from those described in cardiac and cerebral procedures, namely, patient preference and lower rates of access-related complication [7]. For now, it remains our practice to utilize transfemoral access for routine spinal embolization procedures when feasible. However, as TRA-specific tools and collective experience continue to aggregate, it is likely that the TRA will become widely adopted for spinal interventional neuroradiology.
Notes
Ethics Statement
This work was approved by the local institutional review board (IRB) IRB #10-00936. As specific patient information such as age or sex is not included, informed consent for publication was waived.
Author Contribution
Concept and design: MC, CD, and RH. Analysis and interpretation: MC, AB, CD, and RH. Data collection: MC, ES, and RH. Writing the article: MC. Critical revision of the article: MC, ES, AB, and CD. Final approval of the article: MC, ES, CD, and RH. Overall responsibility: MC, AB, CD, and RH.
Fig. 1.
(A) Digital subtraction angiography selective injection of the left T3 segmental artery through the diagnostic catheter demonstrates cranial angulation and hypervascular tumor blush (black straight arrows) involving the T3 vertebral body and adjacent soft tissues. (B) Post-embolization selective injection through the microcatheter (white arrowhead) shows significantly reduced tumor vascularity with intra-arterial coil placement (curved black arrow). The course of the Simmons-type catheter from the trans-radial approach is demonstrated by curved white arrows.

Fig. 2.
TRA using long (130 cm) Penumbra Vert Catheter (A, white curved arrows). DSA roadmap (B) shows near parallel orientation of the left L4 segmental artery origin and the catheter tip angle (white dotted arrows). Over a glidewire, the catheter could easily select the segmental artery (C, tumor blush=black arrows), facilitating successful repeat embolization using PVA particles (D, post-embolization, diminished tumor blush=white arrows). TRA, transradial approach; DSA, digital subtraction angiography; PVA, polyvinyl alcohol.
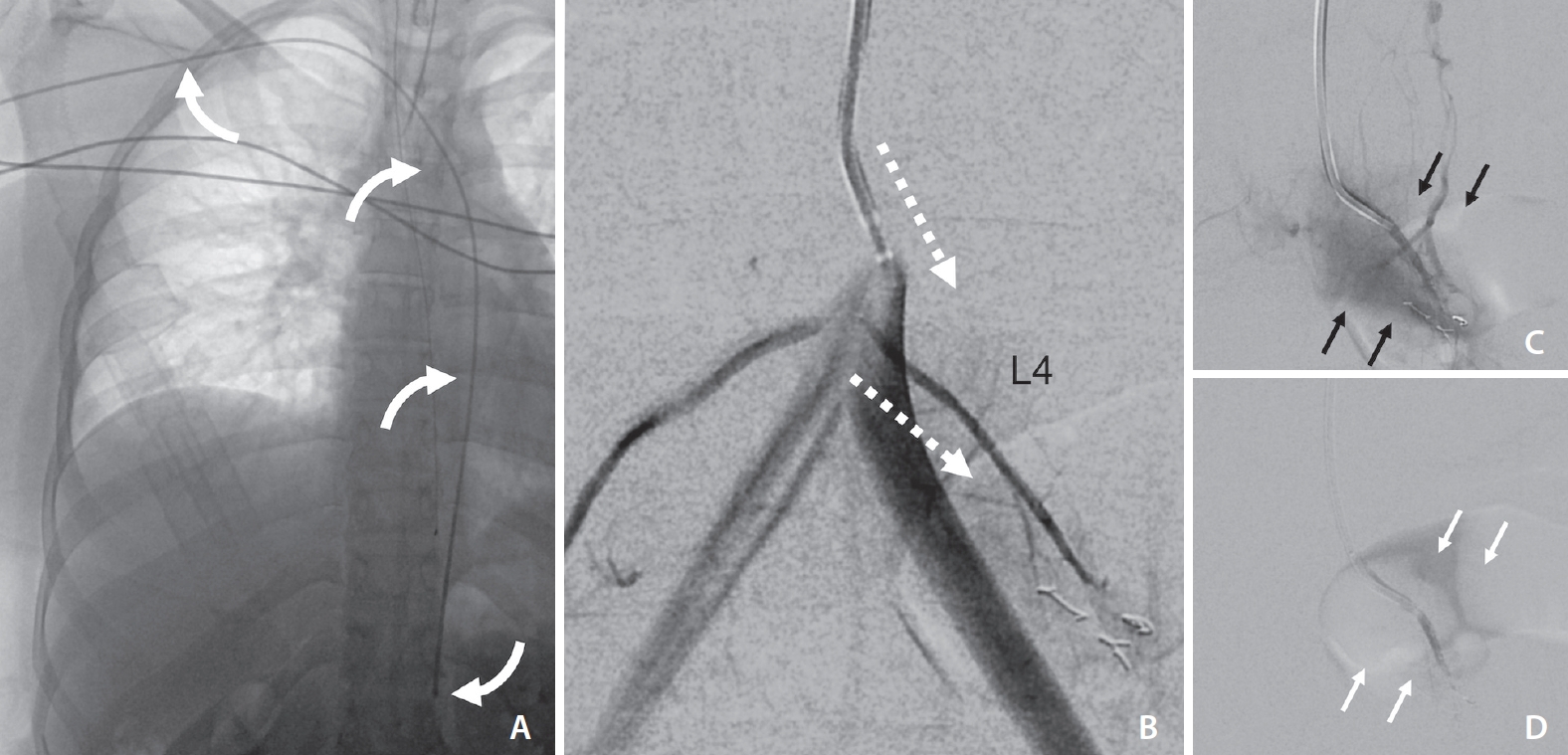
Fig. 3.
Summary of “inverse” catheter geometry and strategies for spinal radicular artery selection with 4 common aortic catheter types. The Simmons-type catheter (A) was used to access the left T3 segmental artery and may be favorable for many upper and mid-thoracic lesions. The C2 (“Cobra”) catheter (B) has a cranially-directed tip which may be advantageous for upper thoracic segmental arteries. The Vertebral catheter shape (C) affords less support in the aorta lumen but may be optimal for caudally-directed lumbar segmental arteries. The Mikaelsson catheter (D) offers the most “neutral” tip-angle and would therefore function similarly from transradial and transfemoral approaches, providing the greatest stability at mid-thoracic levels.
REFERENCES
1. Wilson MA, Cooke DL, Ghodke B, Mirza SK. Retrospective analysis of preoperative embolization of spinal tumors. AJNR Am J Neuroradiol 2010;31:656-660.



2. Prabhu VC, Bilsky MH, Jambhekar K, Panageas KS, Boland PJ, Lis E, et al. Results of preoperative embolization for metastatic spinal neoplasms. Neurosurg 2003;98(2 Suppl):156-164.

3. Gellad FE, Sadato N, Numaguchi Y, Levine AM. Vascular metastatic lesions of the spine: preoperative embolization. Radiology 1990;176:683-686.


4. Griessenauer CJ, Salem M, Hendrix P, Foreman PM, Ogilvy CS, Thomas AJ. Preoperative embolization of spinal tumors: a systematic review and meta-analysis. World Neurosurg 2016;87:362-371.


5. Gailloud P. Introduction to diagnostic and therapeutic spinal angiography. Neuroimaging Clin N Am 2019;29:595-614.


6. Narsinh KH, Mirza MH, Duvvuri M, Caton MT Jr, Baker A, Winkler EA, et al. Radial artery access anatomy: considerations for neuroendovascular procedures. J Neurointerv Surg 2021;13:1139-1144.


7. Narsinh KH, Mirza MH, Caton MT Jr, Baker A, Winkler E, Higashida RT, et al. Radial artery access for neuroendovascular procedures: safety review and complications. J Neurointerv Surg 2021;13:1132-1138.


8. Haynes J, Nossek E, Shapiro M, Chancellor B, Frempong-Boadu A, Peschillo S, et al. Radial arterial access for thoracic intraoperative spinal angiography in the prone position. World Neurosurg 2020;137:e358-e365.


9. Kühn AL, de Macedo Rodrigues K, Singh J, Massari F, Puri AS. Distal radial access in the anatomical snuffbox for neurointerventions: a feasibility, safety, and proof-of-concept study. J Neurointerv Surg 2020;12:798-801.


10. Orru E, Tsang COA, Klostranec JM, Pereira VM. Republished: transradial approach in the treatment of a sacral dural arteriovenous fistula: a technical note. J Neurointerv Surg 2019;11:e4.


11. Knox JA, Alexander MD, McCoy DB, Murph DC, Hinckley PJ, Ch’ang JC, et al. Impact of aortic arch anatomy on technical performance and clinical outcomes in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2020;41:268-273.










