Arterial Wall Imaging in Symptomatic Carotid Stenosis: Delayed Enhancement on MDCT Angiography
Article information
Abstract
Objective
To evaluate progressive enhancement in the carotid arterial wall overlying plaque in the symptomatic side for patients with cerebrovascular symptoms until delayed phase using MDCTA.
Materials and Methods
Twenty-one patients (all men; ages, 49-82 years; mean, 67.8 ± 8.4 years) with recent stroke and severe extracranial carotid stenosis were retrospectively analyzed. Pre-, early- and delayed phase images of MDCTA were obtained, and Hounsfield units (HU) of carotid walls were measured. We also measured HU of the asymptomatic contralateral carotid arterial wall for comparison. Friedman's test and Wilcoxon signed-rank test were used to evaluate the differences between groups.
Results
The averaged HU of the carotid wall in the symptomatic side was higher on the delayed phase (65.8 ± 14.2 HU) compared to early arterial phase (54.2 ± 12.6 HU). The averaged HU difference of wall enhancement between pre-contrast and delayed phase (28.0 ± 14.8 HU) was significantly higher than the between pre-contrast and early arterial phase (16.4 ± 12.1 HU) with P < 0.05. In analysis of the contralateral asymptomatic side, the HU difference between pre-contrast and delayed phase (15.5 ± 12.0 HU) showed no significant higher value than between pre-contrast and early arterial phase (14.9 ± 10.9 HU).
Conclusion
The pronounced enhancement of the carotid wall in the delayed phase on MDCTA was demonstrated in symptomatic patients with severe internal carotid artery stenosis. In the future, we need more comparative studies to verify this finding as one of risk stratification.
Extracranial carotid stenosis accounts for 15-20% of all ischemic stroke, and the degree of luminal stenosis has been considered an important risk factor for stroke and a standard parameter for stenosis severity caused by atherosclerosis, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [1]. However, in recent years, there has been more emphasis on morphology and composition of plaque, giving rise to the concept of vulnerable plaque [23].
Noninvasive image modalities, such as ultrasound, positron emission tomography (PET), magnetic resonance imaging (MRI), and multi-detector-CT angiography (MDCTA), have demonstrated that certain morphological features of vulnerable plaque, such as a large lipid-rich necrotic core, intraplaque hemorrhage, neovascularization, inflammation, or thin fibrous caps, are associated with risk of stroke [4]. Among them, hyperplasia of the adventitial vasa vasorum and neovascularization are important features of plaque development, and association between neovascularization and plaque vulnerability has been suggested [5678]. Some authors have indicated that carotid wall enhancement in the delayed phase on dynamic contrast-enhanced MRI is a reliable sign of plaque neovascularization and inflammation linked to increased risk of clinical vascular events [91011]. Recently, Romero et al. [12] demonstrated early arterial CTA enhancement of the ICA wall is significantly more common in symptomatic than in asymptomatic patients with more than 70% ICA stenosis.
The purpose of this preliminary study is to evaluate progressive contrast enhancement carotid artery wall overlying plaque of the symptomatic side in patients with cerebrovascular symptoms until the delayed phase using MDCTA and assess whether there is a statistical association between increase in Hounsfield Unit (HU) of the carotid wall in the delayed phase and cerebrovascular symptoms.
MATERIALS AND METHODS
Patient population
Clinical and imaging data, obtained as part of the standard clinical stroke care at our institution, were retrospectively reviewed with approval of the institutional review board. Between April 2009 and November 2011, 21 patients (all men; ages, 49-82 years; mean, 67.8 ± 8.4 years) with > 70% carotid stenosis on MDCTA (according to NASCET) [13] within 2 weeks from ischemic episode and in whom carotid stent was inserted were enrolled in this retrospective study. The patients were def ined as "symptomatic" based on clinical presentation at admission and positive diffusion-weighted imaging at follow-up during admission. We defined the "asymptomatic" side as the contralateral carotid artery with no relevant correlation to clinical symptoms in a patient.
MDCTA protocol
All patients were scanned from the aortic arch to the head using a 64-multi-detector-row CT scanner (Brilliance 64, Philips Medical Systems, Best, The Netherlands) with a standardized optimized contrast-enhanced CT angiography protocol.
In our protocol for the analysis of carotid arteries, an unenhanced baseline acquisition of the entire carotid artery was performed. An automatic bolus-tracking program was used to start acquisition after contrast injection (370 mg I/ml, Ultravist 370, Bayer Schering Pharma AG, Berlin, Germany). After the start of contrast material injection, the software measured the attenuation value for a region of interest (ROI) within the ascending aorta and scanning started after 3.2 seconds as soon as the threshold of 150 HU was exceeded. The injection rate of contrast media was 6 ml/sec, for a total volume of 100 ml. CT technical parameters included: 120 mAs, 120 kV, a 1-mm slice thickness, 64 × 0.625 mm detector configuration using a pitch of 0.89 for pre-contrast phase; 180 mAs, 120 kV, a pitch of 0.89 for early arterial phase; 100 mAs, 120 kV, a pitch of 0.67 for delayed phase (90 sec after contrast injection). Subsequently, the images were processed at a workstation to create multiplanar reconstruction for analysis of degree of stenosis and plaque morphology. The total effective radiation dose during study averaged about 4.02 ± 0.2 mSv.
Image analysis
We evaluated severity of stenosis according to the NASCET and carotid plaque wall on pre-contrast, early arterial, and delayed phase measured in Hounsfield units (HU). Carotid wall enhancement was considered present if >50% of the carotid wall circumference was enhanced at the level of maximum stenosis with a 10-HU increase.
Bilateral carotid vessels were evaluated for each patient to comparatively evaluate the asymptomatic side. The MDCTA were separately analyzed by two experienced neuroradiologists by consensus. In the contrast-enhanced early and delayed phases, a circular ROI, three to f ive in number and with 1 mm in diameter, in the predominant enhancing area of carotid wall overlying plaque was used to measure HU value at the most stenotic site (Fig. 1). A ROI of the same area as that used in the contrast-enhanced phase was positioned in the same position at carotid artery wall to measure the unenhanced HU value at an equivalent level. In order to measure the degree of enhancement, we calculated the HU difference between pre-contrast and contrast enhanced early arterial and delayed phases. During the image review, the center level was set at 100-150 HU and the window width at 250-350 HU, which allowed for optimal plaque component distinction. Severe calcified components covering the vessel wall were excluded from the ROI.
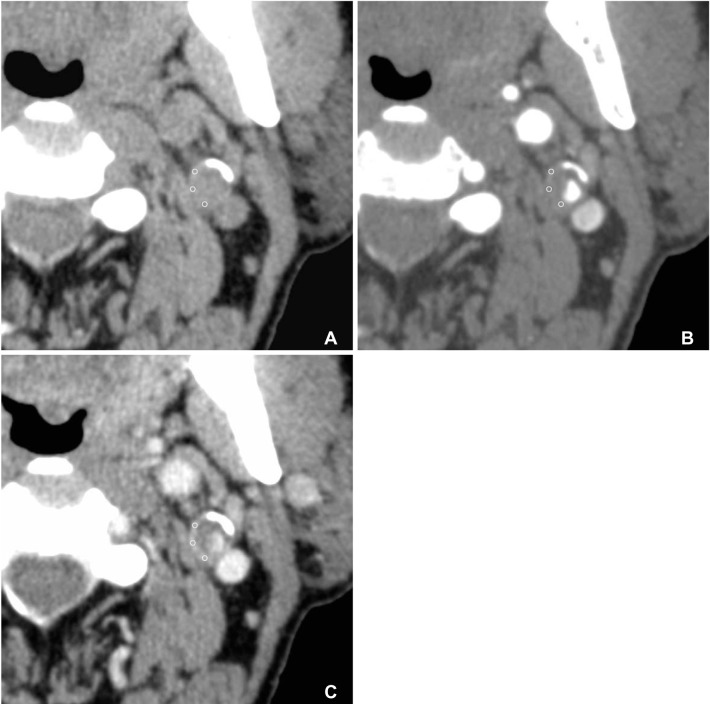
A 75-year-old symptomatic male patient with acute infarctions at the left frontal and parietal lobes. MDCT axial images in the before (A), early arterial (B), and delayed (90 sec) phases (C) after administration of contrast material. The circles indicate the region of interest in the wall of the left proximal internal carotid artery (ICA). The mean HU of wall in each phase measured 58.6 HU, 73.6 HU, and 92 HU, respectively. The HU difference between the delayed (90 sec) and pre-contrast (A) phases was 33.4, while differences between early arterial (B) and pre-contrast (A) phases measured 15.
Statistical analysis
For the analysis of the data, averaged HU values between unenhanced and early arterial and delayed phase were calculated. Friedman's test was used to correlate averaged HU values and the difference of the carotid wall between pre-contrast and contrast-enhanced phases within each symptomatic and asymptomatic group. The Wilcoxon signed-rank correlation test was used to test averaged HU values and difference between symptomatic and asymptomatic groups. Differences were defined as significant at a probability level <0.05. All analyses were performed using the SPSS 15.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
The clinical characteristics of the study population are as follows: hypertension in 19 patients (90.5%); diabetes mellitus in 7 (33.3%); hyperlipidemia in 11 (52.4%). The mean luminal stenosis degree in the symptomatic side measured 82% (range, 70-99%), and the mean luminal stenosis degree in the contralateral asymptomatic side measured 40% (range, 0-99%).
The HU of carotid wall enhancement overlying plaque and statistical analysis in all patients are demonstrated in Table 1. In the symptomatic side of all patients, the mean HU of carotid wall enhancement at the stenotic portion was higher in the delayed phase (65.8 ± 14.2 HU) compared to the early arterial phase (54.2 ± 12.6 HU). The HU difference of carotid wall enhancement between the pre-contrast and delayed phase (28.0 ± 14.8 HU) was significantly higher than between the pre-contrast and early arterial phase (16.4 ± 12.1 HU) with P < 0.05. In contrast, in the asymptomatic contralateral side, the averaged HU difference between the pre-contrast and delayed phase (15.5 ± 12.0 HU) showed no significance higher than between the pre-contrast and early arterial phase (14.9 ± 10.9 HU) with P = 0.05.
Between symptomatic and asymptomatic sides, relative statistically significance existed in the averaged HU value and difference of pre-contrast and delayed phase (28.0 ± 14.8 HU vs. 15.5 ± 12.0 HU, P = 0.008). However, there was no significant correlation in the averaged HU value and the difference between the pre-contrast and early arterial phase between the symptomatic (16.4 ± 12.1 HU) and asymptomatic side (14.9 ± 10.9 HU) with P = 0.823.
DISCUSSION
For all patients in this study, there was a statistically higher HU difference of carotid wall overlying plaque between the pre-contrast and delayed phases than the HU difference between the pre-contrast and early arterial phases demonstrated on the symptomatic side (Fig. 2). This carotid wall enhancement that we observed may be attributed to neovascularization and inflammation. Plaque neovascularization, first described by Koester is thought to participate intimately in the growth and progression of human atherosclerosis [14]. It has previously been demonstrated, by using different imaging techniques, that enhancement of carotid plaque correlated with increase of neovessels within the carotid plaque and that the cause of enhancement is due to increased vascularity of the adventitial vasa vasorum feeding the plaque neovasculature [151617]. Kerwin et al. [11] reported that, on dynamic contrast-enhanced MRI, temporal increase in enhancement on the outer rim or adventitia until delayed phase due to neovascularization is a sign of plaque inflammation and vulnerability linked to increased risk of clinical vascular events. Staub et al. [18] conf irmed pronounced enhancement of the adventitial vasa vasorum on contrast-enhanced carotid ultrasound was associated with clinical events and that the presence of vasa vasorum-derived intraplaque neovascularization was associated with plaque instability. Romero et al. [12] recently reported CTA enhancement of the ICA wall is significantly more common in symptomatic than in asymptomatic patients with severe stenosis and explained it as vasa vasorum noevascularization in the early arterial phase. To our knowledge, this is the first study that examined carotid wall enhancement until the delayed phase using MDCTA and its correlation with a patient's clinical history.

A 71-year-old symptomatic male patient with acute cerebral infarction at left middle cerebral artery territory. MDCTA axial images in the before (A), early arterial (B), and delayed (90 sec) (C) phases after contrast enhancement. Compared to the asymptomatic right side (arrow), the symptomatic left carotid arterial wall overlying the plaque demonstrates more prominent and discrete enhancement in the delayed phase image (arrowheads).
In contrast to our study, recently it has been reported that an increase in HU from the early to delayed phases on MDCTA indicates plaque stability [19]. However, their study analyzed dynamic contrast enhancement of variable plaque components, including fibrous tissue, lipid core, intraplaque hemorrhage, and neovascularization, with free-hand segmentation of the ROI. In contrast, considering heterogeneity in the carotid plaque component, we focused on wall enhancement overlying the carotid plaque, and hypothesized that it would reflect plaque vulnerability more accurately and constantly with respect to adventitial neovascularization. This different approach may explain the different results in our study. Additionally, Saba et al. [20] recently reported that using contrast enhanced MDCTA, contrast plaque enhancement is statistically associated with neovascularization and microvessel density in symptomatic patients who underwent carotid endarterectomy. However, our study analyzed the arterial wall enhancement overlying plaque specifically, and our result may be complementary and allow better understanding of the behavior of vulnerable and non-vulnerable plaque.
Some limitations in this study should be addressed. First, MDCTA leads to ionizing radiation exposure [21]. However, CTA is a routine and adjunctive imaging modality that is obtained as part of the standard of care in a few seconds, without any requirement for a specif ic protocol in acute or chronic cerebrovascular disease. Moreover, MDCTA is a useful and noninvasive modality that gives information about the degree of luminal stenosis, extent of intra-plaque calcification, or ulceration with acceptable range of radiation during our study. Second, our study used contralateral carotid arteries as controls, since it has the advantage of accounting for various factors such as age and cardiovascular risk factors. However, an alternative method could have used asymptomatic age-matched controls with severe atherosclerotic disease. Third, this study did not quantify HU for the entire plaque volume with free-hand segmentation for a ROI because of technical diff iculty and limited spatial resolution. However, we selected the circle segmentation ROI method to measure the carotid wall HU specifically, because we hypothesized this method would be more accurate in measuring the wall in which we are interested. Finally, we could not correlate findings with histologic results, because all patients with severe stenosis were eligible for intervention, which in our hospital includes carotid stenting; and unfortunately, it is not possible to obtain preserved carotid endarterectomy specimens that include an adventitial layer.
In conclusion, the pronounced enhancement of the carotid wall in the delayed phase on MDCTA was seen in symptomatic patients with severe internal carotid artery stenosis. In the future, we need more comparative studies to verify this finding as one of risk stratification.

