Alberta Stroke Program Early CT Score in the Prognostication after Endovascular Treatment for Ischemic Stroke: A Meta-analysis
Article information
Abstract
Purpose
The Alberta Stroke Program Early CT Score (ASPECTS) was devised to quantify the extent of early ischemic changes in the middle cerebral artery territory on brain CT. We performed a systematic review and meta-analysis of studies that presented clinical outcomes and baseline ASPECTS in ischemic stroke patients managed with endovascular methods to validate the use of ASPECTS for risk prognostication.
Materials and Methods
We searched the MEDLINE, EMBASE, and Cochran databases for observational or interventional studies that reported clinical outcomes and baseline ASPECTS in ischemic stroke patients treated with endovascular methods. Data were pooled to perform a meta-analysis for comparisons of clinical outcomes between high and low ASPECTS patients.
Results
A meta-analysis of 13 studies (six observational and seven interventional) revealed favorable outcomes (mRS sore 0-2 at 90 days) for high baseline ASPECTS (odds ratio=2.22; 95% CI: 1.74-2.86).
Conclusion
High ASPECTS is a predictor of favorable outcome after endovascular therapy for ischemic stroke.
Several recent randomized controlled trials (RCTs) that evaluated clinical outcomes of endovascular therapy for acute ischemic stroke (AIS) have shown the benefits of endovascular therapy over intravenous thrombolysis (IVT) [12345]. They suggested it should be accepted as a primary treatment choice for large vessel occlusion in AIS.
Appropriate patient selection for endovascular thrombectomy is critical and continues to be refined. This selection criteria is based on the time-window and baseline brain imaging. It is also important to predict the clinical outcome because baseline infarct volume is a factor associated with improved functional outcomes by recanalization treatment [678].
The Alberta stroke program early CT score (ASPECTS) was devised to quantify early ischemic changes on baseline CT in patients with AIS of the anterior circulation. This 10-point quantitative topographic CT scan score can provide volumetric estimates of the size of cerebral infarction of anterior circulation. In many clinical studies of IVT, Although current data is uncertain relative to ASPECTS providing exclusion criteria for IVT, ASPECTS is a reliable predictor of functional outcomes, having strong correlations with functional outcome, mortality, and symptomatic intracranial hemorrhage (sICH) [910].
To our knowledge, although ASPECTS was originally designed for use with IVT, recent studies indicated its practicability for use with endovascular treatment [111213]. However, previous studies in smaller cohorts are insuff icient to clarify the predictability of baseline ASPECTS in endovascular therapy. Therefore, a comprehensive review is needed to validate the clinical role of ASPECTS.
Our aim was to perform a comprehensive review and meta-analysis of clinical trials that recorded baseline ASPECTS in AIS patients managed with the endovascular method to yield reliable estimates of outcome prognostication of this approach before endovascular therapy.
MATERIALS AND METHODS
Search Strategy
Meta-analyses were undertaken in accordance with guidelines for meta-analysis of observational studies in epidemiology (MOOSE) [14]. Two researchers (C.R., S.S.) identified all published observational or interventional studies that reported clinical outcomes according to baseline ASPECTS in acute ischemic stroke patients treated with endovascular management. We conducted a systematic literature review of PubMed, EMBASE, and Cochrane databases from January 2000 to January 2016. The following key words and entry terms analogous with these were used for searching in relevant combinations by using the Boolean operators OR and AND: “cerebrovascular accident,” “stroke,” “ischemia,” “embolectomy,” “endovascular,” “intraarterial thrombectomy,” and “thrombolysis”. Following the search, articles were then screened by title and abstract for the aforementioned inclusion criteria. The references of included articles were assessed to identify additional potentially relevant studies. The search was restricted to human studies in English.
Inclusion and exclusion criteria
Studies were selected in the primary list as follows: any study that 1) involved AIS patients eligible for endovascular therapy; 2) reported over 20 cases undergoing endovascular therapy; 3) reported baseline ASPECTS of brain imaging modality in AIS patients; and 4) accurately described functional outcomes using a modified Rankin scale (mRS) at 90 days. We excluded studies 1) having duplicated data; 2) reporting mainly patients with posterior circulation stroke; 3) in which ASPECTS was graded based on perfusion imaging only, and 4) lacking appropriate data. A PRISMA flow diagram was used to illustrate the decision-making process regarding the studies.
Data extraction
Three reviewers (C.R., S.P., H.S.) independently extracted data from selected studies that fulfilled the inclusion and exclusion criteria using a standardized form, and all disagreements were resolved by consensus. The following data were collected: report characteristics (first author's name, journal, year of publication); study design (retrospective/prospective analysis, intervention trial or observation, name of cohort, single/two arms, single/multicenter), indication for endovascular therapy, treatment option (the administration of intravenous thrombolytic agent (IVT) before endovascular therapy, type of intra-arterial approach), study sample (sample size, age, sex, baseline National Institutes of Health Stroke Scale [NIHSS], baseline ASPECTS, the occlusive segment, time from symptom onset to recanalization, rate of recanalization); and data regarding and definitions of outcomes (favorable functional outcome, mortality, and sICH). If a study presented outcomes according to dichotomized ASPECTS, we used these data without modification, and if not, ASPECTS was dichotomized into high (>7) and low (≤7) using data extracted for primary analysis, as performed previously [15].
The quality of the included studies was also independently assessed by the two reviewers (C.R., H.S.) using the “Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies,” provided by the National Institutes of Health (NIH) [16] and/or Cochrane Collaboration's tool for assessing risk of bias [17].
Outcome measures
Predictability was assessed by comparisons of outcomes between dichotomized ASPECTS: high and low. The primary outcome of interest was the proportion of patients with favorable mRS scores of 0 to 2 at 90 days post intervention. The secondary outcomes assessed were all-cause mortality and sICH. We calculated, and subsequently pooled in independent meta-analyses, the odds ratios (ORs) with corresponding 95% confidence intervals (CI) for each outcome of interest.
For all meta-analyses, outcomes were pooled using the DerSimonian and Laird random-effects model, with weights calculated by the Mantel-Haenszel method. Heterogeneity between studies was assessed using Cochrane's Q test and I2 statistic, with I2 values exceeding 50% representing significant heterogeneity. Publication bias was evaluated visually by funnel plots or quantified by the Begg-Mazumdar's rank correlation test.
Subgroup analyses and meta-regression were used to determine whether study-related factors could account for heterogeneity. Subgroup analysis was performed according to the study design and included intra-arterial chemical thrombolysis as the primary endovascular treatment option versus mechanical thrombectomy as the primary option. We were able to analyze the impacts of the rate of internal carotid artery (ICA) occlusion, the rate of recanalization, and time from symptom to recanalization for meta-regressions. In order to evaluate the stability of the pooled results, we further conducted sensitivity analyses by removing studies with higher risks of introducing bias.
A further analysis was conducted with ASPECTS dichotomized at very low (0-4) versus intermediate and high (5-10), and dichotomized at indeterminate (5 or 6-7) versus high (8-10). P-values <0.05 were considered significant.
RESULTS
Study selection
A total of 16,831 studies were identified during the initial search. Of these, 13 studies were pertinent to include in this review [34511121318192021222324]. A flow diagram summarizing the literature search is presented in Figure 1.
Study Characteristics
The main methodological and baseline characteristics of included studies are presented in Tables 1 and 2. Three studies were primary reports of RCTs (ESCAPE, REVASCAT, SWIFT-PRIME) that compared the treatment effects of endovascular therapy to those of the current standard therapy for patients with AIS (3-5), and three studies were ancillary analyses of endovascular arms of RCTs (PROACT-II, DEFUSE-II, IMS-III) [182123]. One study used pooled data from two single-arm trials (Penumbra phase II pivotal trial; ClinicalTrials.gov; NCT00334061, and Penumbra Imaging Collaborative Study; ClinicalTrials.gov; NCT00785161) to assess the safety and efficacy of endovascular devices [11]. The other six studies were retrospective reviews of consecutive single-/multicenter case series. No studies were considered to be seriously flawed as per the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (Table 3).

Risk Assessment of Individual Studies Using the National Institute of Health Quality Assessment Tool for Observational Cohort and Cross-sectional Studies
All locations of ischemic strokes were within the anterior circulation distribution. Two studies included only MCA occlusion as inclusion criteria. Median/mean NIHSS and ASPECTS were similar within each study. Of 1,798 participants (nine studies), 74% had IVT before endovascular treatment, and in two studies, patients did not receive intravenous thrombolytic agents before endovascular treatment according to their study protocols. In four studies, intraarterial chemical thrombolysis was the primary choice of endovascular therapy or was given to over a half of the endovascular cohorts. Penumbra suction systems and/or stent retrievers were used as the primary choice for endovascular thrombectomy in nine studies. Eleven studies used non-enhanced CT to score ASPECTS and three studies used MRI. An ASPECTS dichotomy threshold of seven was used in 12 studies and a threshold of six was used in two studies.
Prognosis Predictability
Primary outcome
Meta-analysis of pooled data from the 13 included studies revealed favorable functional outcomes (mRS sore 0-2 at 90 days) in favor of high baseline ASPECTS (OR=2.227; 95% CI: 1.735 to 2.859; P<0.0001) (Fig. 2A). There was no significant heterogeneity among the included studies (Q-statistics, P=0.12; I2 =32.96%). Significant publication bias was not observed (Kendal's tau, 0.000; two-tailed P=1.00) (Fig. 2B).

Clinical outcomes of endovascular treatments in ischemic stroke patients with high ASPECTS vs. low ASPECTS A. Meta-analyses of high ASPECTS (>7) vs. low ASPECTS (≤7) for favorable clinical outcome (modified Rankin scale score 0-2) (A). The relative size of the data markers indicates the weight of each study's sample size. The included trials for functional outcome are divided into subgroups according to study design (prospective randomized controlled or single-arm trials and observational case series). B. A funnel plot with all points evenly distributed on both sides of the solid vertical line indicates no publication bias. C. Subgroup analysis of favorable outcomes of endovascular treatments in ischemic stroke patients with high ASPECTS vs. low ASPECTS. The included trials for functional outcome are divided into subgroups according to the methods of endovascular therapy (dominant intraarterial thrombolysis and dominant intraarterial thrombectomy). One study (23) which did not clearly present the endovascular methods was excluded from subgroup analysis for endovascular methods. D. Scatterplots of the relationship between the rate of ICA occlusion and log odds ratio. The size of the bubbles indicates the weight of each study in the meta-analysis. The trend line indicates the degree to which the log odds ratio decreases as the rate of ICA occlusion in subjects increases.
Subgroup analyses of the seven prospective trials (OR=2.154; 95% CI 1.685 to 2.754; P<0.0001; Q-statistics, P=0.74; I2=0.00%) and six retrospective case series (OR=2.422; 95% CI, 1.764 to 3.326; P<0.0001; Q-statistics, P<0.05; I2=64.37%) also demonstrated favorable outcomes in favor of high ASPECTS (Fig. 2A). There were no significant differences between subgroups (Q-statistics, P=0.567). Subgroup analyses of four studies that included intra-arterial thrombolysis as the dominant endovascular treatment option also showed that initial high ASPECTS was associated with stronger odds for good outcomes (OR=2.638; 95% CI, 11.971 to 3.531; P<0.0001; Q-statistics, P=0.06; I2=59.67%). Pooled analyses of eight studies that used new generation devices (suction thrombectomy and stent retriever) for thrombectomy yielded the same results (OR=2.074; 95% CI, 1.580 to 2.721; P<0.0001; Q-statistics, P=0.41; I2=2.06%). There were no significant differences between the subgroups (Q-statistics, P=0.236) (Fig. 2C).
Meta-regression analysis demonstrated that the log odds ratios between high and low ASPECTS arms decreased with increases in the ICA occlusion rate (regression coefficient=−0.048; 95% CI, −0.078 to −0.017; P = 0.002) (Fig. 2D) in 11 studies, but did not identify any correlations with functional outcome on the basis of time to recanalization or the rate of pre-interventional IVT.
Exclusion sensitivity analysis demonstrated that pooled estimates did not change with the exclusion of any one study for either effect size measure. Pooled estimates of good outcomes were not significantly different, with the exclusion of three studies that used MRI to score ASPECTS, four studies that did not have upper age limits for inclusion, and two studies that excluded subjects with ICA occlusions.
Secondary outcomes
Mortality was extracted from 1,085 patients of seven studies, and sICH was from 1,235 patients of eight studies. Mortality and sICH were significantly lower (OR=2.178; 95% CI, 1.530 to 3.100; P<0.0001; Q-statistics, P=0.32; I2=14.17%; and OR=2.133; 95% CI, 1.510 to 3.015; P<0.0001; Q-statistics, P=0.35; I2=10.59%) (Fig. 3A, B) in high ASPECTS than low ASPECTS.

Mortality and symptomatic intracranial hemorrhage of endovascular treatments in ischemic stroke patients with high ASPECTS vs. low ASPECTS.
A. Mortality at 90 days.
B. Symptomatic intracranial hemorrhage.
In four studies, dichotomization of ASPECTS as very low (≤4) versus intermediate to high (>4) was available for a total of 1,062 patients. Meta-analysis demonstrated good outcomes in favor of intermediate to high ASPECTS (OR=4.077; 95% CI, 1.884 to 8.825; P<0.0001; Q-statistics, P=0.05; I2=60.75%). Dichotomization into intermediate (5 or 6-7) and high ASPECTS (8-10) was available in nine studies. Meta-analysis demonstrated good outcomes in favor of high ASPECTS (OR=1.880; 95% CI, 1.3025 to 2.713; P<0.0001; Q-statistics, P<0.05; I2=53.62%) (Fig. 4).
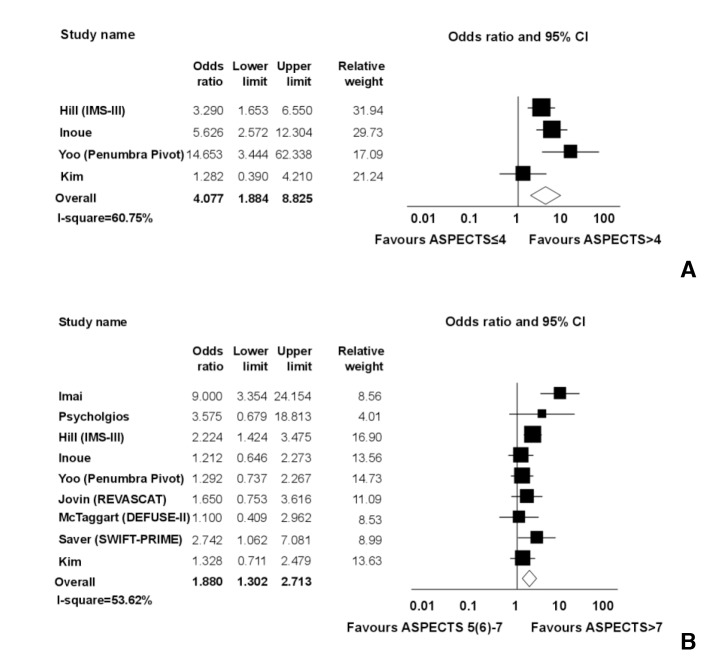
Comparison among very low, intermediate, and high ASPECTS.
A. Favorable outcome (modified Rankin scale score 0-2 at 90 days) of endovascular treatments in ischemic stroke patients with ASPECTS ≤4 vs. ASPECTS >4.
B. Favorable outcome of endovascular treatments in ischemic stroke patients with intermediate ASPECTS (5 or 6 to 7) vs. high ASPECTS (8-10).
DISCUSSION
The meta-analyses showed that a high ASPECTS favored a good clinical outcome after endovascular therapy in comparison with a low ASPECTS. This supports that baseline ASPECTS is a reliable predictor of prognosis in patients with AIS undergoing endovascular therapy, as noted in several individual studies [182125]. Additional subgroup and sensitivity analyses revealed that the primary endpoint of pooled data was not affected by potential biases, such as study design, imaging modality, or endovascular therapy method.
Because the baseline infarction volume is an essential factor affecting clinical results in AIS along with the time-window [26], many researchers have used ASPECTS to assess the efficiency of the treatment modality. In IVT of AIS, the prognostic value of ASPECTS was established in clinical trials with large cohorts, showing a linear correlation between ASPECTS and prognosis [10]. Dichotomous ASPECTS categories were also independent predictors of functional outcomes and sICH [15]. However, ASPECTS was not proven to modify the effectiveness of IVT in terms of improved functional outcomes in two randomized trials compared to placebo [2728], which does not support the exclusion of patients from IVT based on ASPECTS.
Unlike IVT, the present study indicates that ASPECTS can identify candidates who are suited for endovascular therapy in AIS and can help determine the best treatment option without delaying therapy. In this meta-analysis, the pooled data showed heterogeneity across enrolled studies, which was explained by treatment option, intraarterial thromobolysis versus intraarterial thrombectomy. Such differences in endovascular method have also been suggested to be a confounding factor in previous meta-analyses comparing the effectiveness of endovascular therapy vs. medical treatment of ischemic stroke (29, 30). Because the present analysis used a dichotomous classification of ASPECTS and many of enrolled studies did not provide cut-off values of ASPECTS for candidates of endovascular therapy but instead used the one-third rule, we were unable to specify the lowest ASPECTS value associated with therapeutical benef its after endovascular therapy. Therefore, further studies should be conducted to identify cut-off values of ASPECTS for selecting patients likely to benefit from endovascular therapy.
Although ASPECTS was originally designed for use with non-enhanced brain CT, recent studies assessed its practicability with advanced brain imaging methods, such as perfusion CT and MRI, due to limitations associated with interrater reliability, predictability, and sensitivity for early ischemic lesions when assessed using CT-ASPECTS compared with MRI-ASPECTS [23]. However, CT has the advantage of being an easy approach and saving the time to treatment, and has stronger evidence through many clinical trials. Therefore, CT-ASPECTS will not be replaced by alternative scoring systems in clinical practice within the next few years. The lower reliability of CT ASPECTS should be overcome by training clinicians who assess patients initially after admission to the hospital [31]. In this study, meta-regression showed that the rate of ICA occlusion was negatively correlated with odds ratios in favor of high ASPECTS. This f inding suggests that lower recanalization rate, procedural difficulty, and weaker collateral supply in ICA occlusion are factors that decrease the likelihood of good outcomes, despite initial small volumes of infarction [32].
Our study has several potential limitations. First, because baseline ASPECTS was not randomized by researchers in most studies, this manuscript was described based on guidelines for meta-analyses of observational studies. Second, our results could be constrained by the unclear risk of bias owing to incomplete data in a few studies.
Our meta-analysis of pooled data, including prospective trials and observational studies, provides high-level evidence of outcome prognostication of dichotomized ASPECTS in endovascular therapy for ischemic stroke. Scoring the baseline ASPECTS would enhance the selection of candidates for endovascular therapy by predicting prognosis in the management of ischemic stroke.
Acknowledgement
We thank Dr. Woong Yoon for providing raw data referenced in his article (ref. 24).





