Temporal Changes in Care Processes and Outcomes for Endovascular Treatment of Acute Ischemic Stroke: Retrospective Registry Data from Three Korean Centers
Article information
Abstract
Background and Purpose
The purpose of the current study is to evaluate the influence of temporal patterns related to the availability of new endovascular treatment (EVT) devices on care processes and outcomes among patients with AIS.
Materials and Methods
We enrolled 720 consecutive patients (January 2011 to May 2016) in a retrospective registry, ASIAN KR, from three Korean hospitals, who received EVT for acute ischemic stroke (AIS) caused by cervicocephalic arterial occlusions. We performed period-to-period analyses based on stent retriever reimbursement and the availability of second-generation direct-aspiration devices (Period 1: January 2011–July 2014 vs. Period 2: August 2014–May 2016); time metrics and outcomes were compared when the onset-to-puncture time was <720 min among patients with EVT for intracranial occlusion.
Results
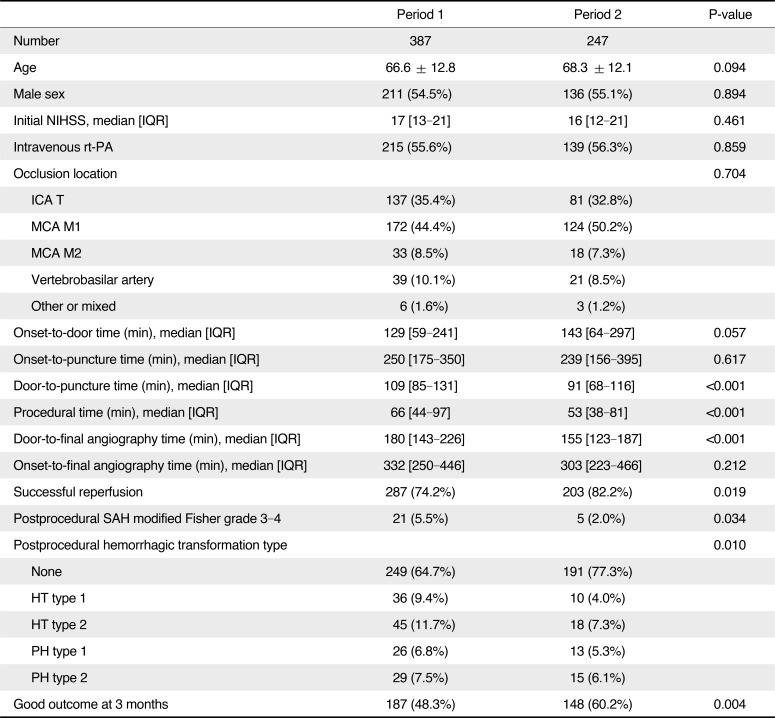
Period 2 had better post-EVT outcomes (3-month modified Rankin Scale 0–2 or equal to prestroke score, 48.3% vs. 60.2%, P=0.004), more successful reperfusion rates (modified Treatment In Cerebral Ischemia 2b–3, 74.2% vs. 82.2%, P=0.019), fewer subarachnoid hemorrhages (modified Fisher grade 3–4, 5.5% vs. 2.0%, P=0.034) and lower hemorrhagic transformation rates (any intracerebral hemorrhage, 35.3 vs. 22.7%, P=0.001) than Period 1. Compared to Period 1, Period 2 had a shorter door-to-puncture time (median 109 vs. 90 min, P<0.001), but longer onset-to-door time (129 vs. 143 min, P=0.057).
Conclusion
Recent temporal improvements in post-EVT AIS outcomes in Korea are likely due to a combination of enhanced hospital care processes and administration of newer thrombectomy devices.
New thrombectomy devices such as stent retrievers or direct clot aspiration devices have enabled great progress in the reperfusion rate for intracranial large artery occlusions causing acute ischemic stroke.123 Recent major randomized controlled trials (RCTs) using these new devices have now established endovascular treatment (EVT) as the standard treatment for patients presenting with emergent intracranial large vessel occlusions.45678 In response to these trials, the clinical practice guidelines were updated to include EVT as the standard care for acute ischemic stroke worldwide.9101112 However, these major RCTs only included those patients who had anterior circulation occlusion, with a small core, good collateral circulation, and/or large penumbra zone. In addition, Asian populations were rarely included in the RCTs.
To date, studies on the use of EVT in patients of Asian ethnicity have been based mainly on single-center retrospective data. Here, we performed a real-world analysis utilizing a multicenter registry consisting of consecutive patients, whose clinical data were utilized and imaging data were evaluated through standardized core lab evaluations.13 The Acute Stroke due to Intracranial Atherosclerotic occlusion and Neurointervention - Korean Retrospective registry (ASIAN KR registry) includes consecutive patients who received EVT at three comprehensive stroke centers in Korea.
The aim of the current study was to assess temporal trends in the use and outcomes of EVT in patients with acute ischemic stroke. In addition, we evaluated whether additional factors beyond the application of new devices would affect the clinical outcomes of EVT by using a period-to-period analysis.
MATERIALS AND METHODS
Study design
This is a retrospective descriptive study. The ASIAN KR is an observational registry of consecutive patients aged 18 or older in whom an endovascular revascularization procedure was attempted for emergent treatment of acute ischemic stroke owing to intracranial and/or extracranial large vessel occlusion. Patient data from three comprehensive stroke centers (Ajou University Hospital [center A, Suwon], Kyungpook National University Hospital [center B, Daegu], and Keimyung University Dongsan Hospital [center C, Daegu]) in South Korea were gathered. Patients who visited centers A and B between January 2011 and February 2016 were included, as were patients who visited center C between January 2011 and May 2016. The database was approved by the Institutional Review Board of each hospital for the registry study. The need for informed consent was waived owing to the retrospective nature of the present study.
Protocols for patient selection
All three centers use noncontrast computed tomography (CT) and CT angiography for baseline imaging. Patients who met the criteria for intravenous (IV) recombinant tissue plasminogen activator (rt-PA) within 3 to 4.5 hours of onset were treated with the infusion. If a large artery occlusion corresponding to the stroke signs was observed on CT angiography, the patients were brought to the angiography suite for EVT. Patients in whom large artery occlusions were identified despite IV rt-PA treatment, those who were ineligible to receive IV rt-PA, or patients who were outside the IV rt-PA time window were considered candidates for EVT. If the onset-to-puncture time was expected to be >6 h, the decision to perform EVT was based on multimodal magnetic resonance imaging (MRI), although the protocols are somewhat varied among the three centers. Patients who did not receive EVT owing to spontaneous recanalization were excluded from the registry. The type of EVT procedure was selected at the discretion of the treating interventionist.
EVT methods
For intracranial mechanical thrombectomy, the direct-aspiration method, stent retrieval technique, or a combination of both methods was used. The direct-aspiration method refers to a forced arterial suction technique (FAST) that uses the Penumbra system (Penumbra Inc., Alameda, CA, USA).14 The second-generation direct-aspiration system was defined as the Penumbra MAX system. In the stent retrieval technique, the clot is removed by capturing the thrombus and bringing it out of the body, using stent retrievers such as Solitaire AB/FR (Medtronic, Irvine, CA, USA) or Trevo (Stryker, Kalamazoo, MI, USA).1215 Balloon guide catheters such as Cello (Fuji System Corp., Tokyo, Japan) or Optimo (Tokai Medical Products, Aichi, Japan) were used to prevent clot migration by proximal balloon inflation while performing direct aspiration or stent retrieval.16 Balloon guide catheters were occasionally used for the remote aspiration method by inflating the balloon and aspirating the guide catheter itself.1718 Intracranial or extracranial angioplasty and/or stenting was sometimes performed at the discretion of physicians if necessary.
De-identification and data collection
In each center, clinical data were recorded in a spreadsheet database with center-specif ic patient identif ication numbers. After their identification numbers and names were de-identified, all data were combined. The clinical information included demographics, risk factors, previous antithrombotic and anticoagulant use, premorbid modified Rankin Scale (mRS) scores, and initial National Institute of Health Stroke Scale (NIHSS) scores. Patients' initial vital signs and routine laboratory data were also collected. Additionally, center-reported imaging results that were used for making therapeutic decisions and identifying intra-arterial therapeutic targets, as well as the devices and methods used for EVT, were included. The clinical outcome was defined by the patients' 3-month mRS scores, which were achieved by each center. A good outcome was defined as a 3-month mRS score of 0–2 or a score equal to the prestroke mRS score if the premorbid mRS score was ≥3.
Core laboratory imaging evaluations
For consistent grading of the multicenter data, core laboratory imaging evaluations were performed. All computed tomography or magnetic resonance imaging data and interventional angiographic data were collected and sent to a core laboratory (Department of Radiology, Ajou University Medical Center, Suwon, South Korea), and then each imaging modality was sent to a designated investigator. After blinding the clinical data and other information, imaging analyses were performed by stroke neurologists, neuroradiologists, and neurointerventionalists with expertise in acute stroke management. The initial large vessel occlusion location was identified on baseline angiography images (S.J.L.). The intracranial occlusion location was classified as internal carotid artery terminus or bifurcation, middle cerebral artery (MCA) M1, MCA M2, or vertebrobasilar artery irrespective of extracranial occlusions. Other or mixed type occlusions were also classified, respectively. The coexistence of relevant extracranial artery involvement was documented separately. By analyzing the angiographic data, intra-arterial treatment targets independent from the initial occlusion sites (Y.H.H.) and the postprocedural reperfusion (modified Treatment In Cerebral Ischemia [mTICI]) grade were identified (Y.H.H. & J.S.L.).19 Successful reperfusion was defined as an mTICI grade of 2b–3.20 The presence of postprocedural hemorrhagic complications was evaluated on brain CT images in most cases or on gradient recalled echo sequence MR images for CT-ineligible cases at around 5 to 7 days after onset. Hemorrhagic transformations were classified according to criteria defined by the European Cooperative Acute Stroke Study (S.I.S.).21 Subarachnoid hemorrhage (SAH) was classified in accordance with the modified Fisher scale (S.I.S.).22 Serious postprocedural hemorrhagic complications were defined as parenchymal hematoma type 2 and/or a modified Fisher grade of 3–4 for SAH. If inconsistencies among the variables were noted, a consensus was reached among the investigators through a full evaluation of all imaging data.
Analyses and statistics
The present analysis first focused on describing the demographics and outcomes of the overall ASIAN KR data. Next, to evaluate which factors contributed to the temporal improvement in clinical outcomes, in patients whose intracranial arterial occlusions were treated by endovascular treatment and whose onset-to-puncture time was <720 min, period-to-period analyses (period 1: January 2011 to July 2014 vs. period 2: August 2014 4 Neurointervention 13, March 2018 Jin Soo Lee, et al. to May 2016) were performed based on the times at which stent retrievers were reimbursed and second-generation direct-aspiration devices became available in Korea. The first generation direct-aspiration systems (Penumbra aspiration catheters) were used in the period 1 whereas the second generation direct-aspiration systems (Penumbra MAX) were used in the period 2. Time metrics, including the onset-to-door, door-to-puncture, and puncture-to-final angiography times, and postprocedural and clinical outcomes were compared between the two periods. Probability of good outcomes was retrieved after adjusting major confounding factors, which included age, sex, premorbid mRS, initial NIHSS score, intracranial occlusion location, infusion of IV rt-PA, onset-to-puncture time, successful reperfusion and serious postprocedural hemorrhagic complications. Regression lines of period 1 and 2, respectively, drawn on scattered plot for the probability of good outcomes and onset-to-puncture time. Additionally, predictors of good outcomes regarding time metrics were determined without period differentiation. Statistical significance was set at P < 0.05. All statistical analyses were performed with SPSS version 22.0 (IBM, Chicago, IL, USA). Graphs were drawn with Excel software (Microsoft, Redmond, WA, USA).
RESULTS
Data from the ASIAN KR registry
A total of 720 patients (center A: n = 199, center B: n = 348, and center C: n = 173) were included in the entire cohort (Table 1). The 3-month mRS score was missing for one patient. MCA M1 (331 cases [46.0%]) and internal carotid artery terminus (248 cases [34.4%]) occlusions were the major occlusion sites. Overall, intracranial EVT was performed in 702 cases while extracranial EVT was performed in 73 cases. The mean age of the cohort was 67.5 ± 12.3 years, with a median NIHSS score of 16 (interquartile range [IQR]: 12–21). The median onset-to-puncture time was 266 (IQR: 176–450) min, and intravenous tissue plasminogen activator was infused in 374 (51.9%) patients. All patients underwent EVT, and successful reperfusion was achieved in 555 (77.1%) patients. Serious postprocedural hemorrhagic complications were encountered in 72 (10.0%) patients, and a good outcome at 3 months was achieved in 370 (51.5%) patients.
Annual trends in intervention methods
The cohort included patients who were enrolled over a 5-year span. Since 2011, the overall frequency of patients enrolled for emergent EVT continuously increased (Fig. 1A). In terms of the number of total cases treated, the frequency of using the direct-aspiration system remained unchanged (Fig. 1B), while the frequency of stent retriever use continued to increase annually (Fig. 1C). The number of cases in which balloon guide catheters were used increased (Fig. 1D). The percentage of patients with successful reperfusion or complete reperfusion increased annually (Fig. 2A), and the clinical outcomes improved annually (Fig. 2B).

Annual trends in intervention method. (A) The total number of endovascular treatment (EVT) cases increased annually at the three centers. The white bar indicates EVT cases performed in 2016 that were not included in the ASIAN KR registry. (B) The use of the direct-aspiration system remained similar throughout the study period. (C) The use of the stent retriever system rapidly increased over the study period. (D) The use of balloon guide catheters increased over the study period.
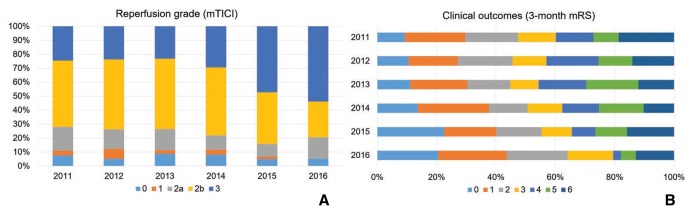
Outcomes per year. (A) The reperfusion grade improved annually throughout the study period (P < 0.001). Complete reperfusion improved considerably in the last 2 years. (B) Clinical outcomes are in line with the reperfusion grade. The percentage of good outcomes progressively improved over the last 3 years (P = 0.097).
Temporal improvements in EVT outcomes
The changes in interventional practices and outcomes were determined by dividing the total study period into two periods among patients who had intracranial large artery occlusions treated by EVT and whose onset-to-puncture time was within 720 min (n = 634) (Table 2). The baseline characteristics such as age, sex, initial NIHSS score, and onset-to-puncture time did not significantly differ between the periods; however, in terms of the time metrics, compared to period 1, the door-to-puncture time was shorter (median 109 [IQR 84–131] vs. 91 [68–116], P < 0.001) while the onset-to-door time was longer (129 [59–241] vs. 143 [64–297], P = 0.057) in period 2. Compared to period 1, successful reperfusion was achieved in a significantly greater percentage of patients in period 2 (74.2% vs. 82.2%, P = 0.019), and the total procedure time from puncture to the final angiography was shorter in period 2 (66 [44–97] vs. 53 [38–81], P < 0.001), as was the door-to-final angiography time (180 [143–226] vs. 155 [123–187], P < 0.001). Moreover, the overall rate of hemorrhagic transformation was reduced (P = 0.010) in period 2, as was the rate of thick SAH (modified Fisher grade 3–4) (5.5% vs. 2.0%, P = 0.034). A good outcome at 3 months was significantly more common in period 2 than it was in period 1 (48.3% vs. 60.2%, P = 0.004). Scattered plot for relationship of onset-to-puncture time and good outcomes showed a different pattern of regression line between period 1 and 2 (Fig. 3). The probability was similarly maintained in both groups until around 400 minutes of onset-to-puncture time; however, after 400 minutes, the probability was steeply reduced in the period 1 whereas the probability was gently reduced in the period 2.
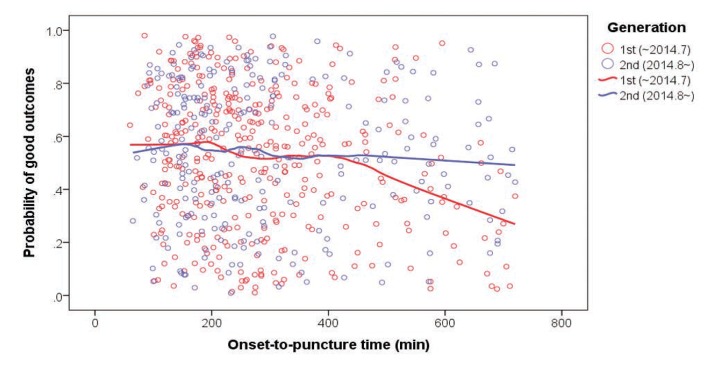
Relationship of onset-to-puncture time and good outcome (modified Rankin Scale score 0–2 at 3 months). The probability of good outcomes was similarly reduced until around 400 minutes of onset-to-puncture time; however, the declining tendency appeared to be steeper in period 1 compared to period 2 after 400 minutes.
Relationship of the time metrics to good outcomes
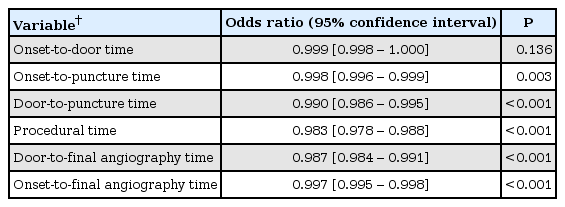
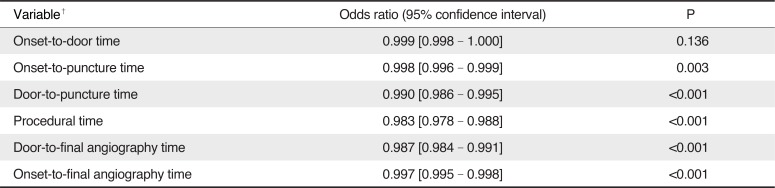
Of the patients who had intracranial large artery occlusions treated by EVT and whose onset-to-puncture time was within 720 min, the in-hospital time metrics, including the door-to-puncture time (0.990 [95% CI, 0.986–0.995], P < 0.001), procedural time (0.983 [95% CI, 0.978–0.988], P < 0.001), and door-to-final angiography time (0.987 [95% CI, 0.984–0.991], P < 0.001), were independent predictors of good outcomes, respectively in separate logistic regression analyses, after adjusting for age, sex, premorbid modified Rankin Scale score, initial NIHSS score, occlusion location, infusion of IV rt-PA, successful reperfusion, and serious hemorrhagic complications; however, the onset-to-door time was not associated with good outcomes (0.999 [95% CI, 0.998–1.000], P = 0.136) (Table 3).
DISCUSSION
The ASIAN KR registry is an observational, multicenter (three comprehensive stroke centers in Korea) registry that is focused on obtaining information about the recent trends in EVT for patients with large arterial occlusions. The current study addresses the clinical, imaging, and procedural factors, and outlines the temporal changes in procedural modalities and outcomes. Overall, we found that successful reperfusion was achieved in 74.2% to 82.2% of patients, and a good outcome was achieved in 48.3% to 60.2% of patients from period 1 to period 2. The results of our analysis are comparable to the results of recent successful RCTs in terms of the recanalization rate and good outcomes at 3 months,45678 although our time window was somewhat longer and included posterior circulation and extracranial occlusion sites. Our real-world data collected from a more-diverse population than those examined in the existing RCTs show that the outcomes of EVT in an Asian population were consistent with those identified by the RCTs. Hence, we believe that the results from the ASIAN KR registry do not deviate from contemporary trends.
In terms of the EVT methods used during the study period, mechanical thrombus removal was the primary method in almost all patients. As illustrated in Fig. 4A, the Penumbra system was commonly used early in our study period, as it was approved in November 2008 and reimbursed in August 2009 in Korea. The second-generation Penumbra system was approved in July 2014 and its reimbursement was maintained from the first generation. The use of stent retrievers continuously increased throughout the study period, reaching its peak in 2015 with the success of RCTs. Solitaire AB has been approved for stent-assisted coiling since May 2010 and was reimbursed in January 2011. The Solitaire FR was approved for mechanical thrombectomy in April 2013 and reimbursed in August 2014. Trevo was approved for mechanical thrombectomy in December 2012 and reimbursed in August 2014. In most centers, if stent retrievers failed as the first option for mechanical thrombectomy, then the direct-aspiration technique was used as a second option, and vice versa. However, in the earlier period, before Solitaire FR was approved, stent retrieval was mostly a rescue treatment following the direct-aspiration technique. We also observed that the use of balloon guide catheters became more frequent over the study period. Since balloon guide catheters can prevent distal embolization of the thrombus,16 it was frequently used with both the direct-aspiration system and stent retriever system, proving its utility. The balloon guide catheter was introduced in early 2011 but was not widely available at that time. Balloon guide catheters were f inally reimbursed in October 2015, and their use has since increased. The application of such high-performance devices likely contributed to the temporal improvement in reperfusion rates and to the reduction in procedural times, which in turn improved the clinical outcomes.
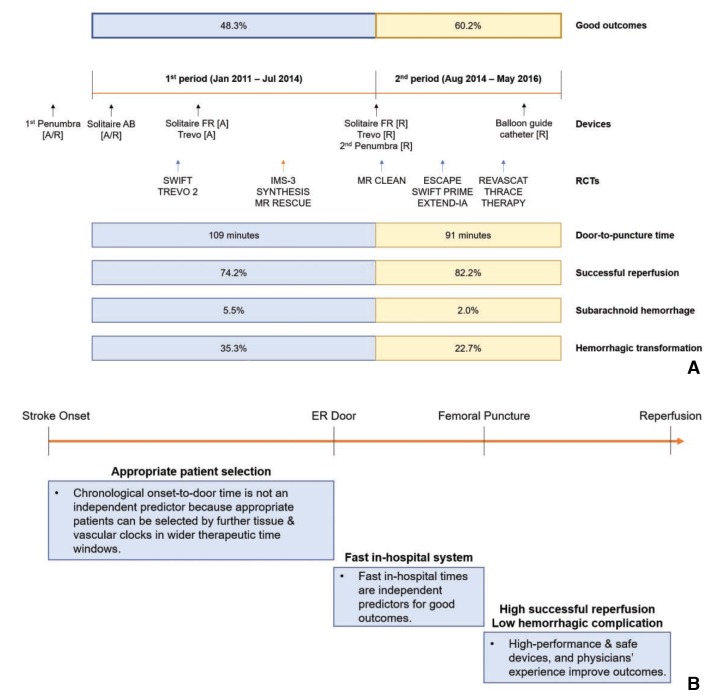
Summary and highlights of the current study. (A) Launches of thrombectomy devices in Korea, presentation of new RCTs overseas, and temporal improvement in endovascular treatment outcomes between period 1 and 2 in the current study. (B) Outcome-improving factors among patients with acute ischemic stroke due to intracranial large artery occlusion who underwent endovascular reperfusion treatment. To summarize the current study, better outcomes were related to fast in-hospital care systems, as represented by reduced door-to-puncture times, and low hemorrhagic complication rates, which resulted from physicians' experiences and the use of new, high-performance devices. Although factors regarding patient selection were not specified in the current study, appropriate patient selection might widen the therapeutic time window without negatively affecting the clinical outcomes. RCT, randomized control trial; [A], approved; [R], reimbursed; ER, emergency room.
Beyond the use of newer thrombectomy devices, several other factors may have contributed to the temporal improvement in clinical outcomes that we observed in period 2 of our study compared to period 1. First and foremost is the shortened door-to-puncture time. Although the onset-to-puncture time was similar between the two periods, the door-to-puncture time was significantly shortened in the later period. Regarding the association of reduced door-to-puncture time with improved clinical outcomes, this finding is consistent with the results of a recent meta-analysis of five RCTs (HERMES pool). In that study, regarding clinical outcomes, a time-by-treatment group interaction was observed in the door-to-puncture time but not in the onset-to-door time.23 Based on our results and the existing literature, it seems that most comprehensive stroke centers have focused on improving their in-hospital care systems.24 These efforts probably contributed to the better outcomes we observed in the later period. Moreover, the shortened times likely represent high institutional performances. The onset-to-door time, which was not associated with clinical outcomes, may be related to more appropriate patient selection and wider therapeutic time windows. The successful results of very recent RCTs, from which could extend time window up to 24 hours of puncture time from symptom onset support our results.2526
Second, the rate of post-procedural hemorrhagic transformation was significantly reduced. The risks of postprocedural hemorrhagic transformation are mainly influenced by not only the chronological clock (delayed treatment time) but also the tissue clock (large infarct core) and vascular clock (poor collateral/ischemic penumbral status or blood-brain barrier breakdown).2728 Attending physicians must quickly decide whether to apply EVT based on these clocks, which requires experience. In the later period of the current study, attending physicians may have been guided by the targeted inclusion criteria used in recent RCTs. Soon after the start point of period 2 in our study, the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands, which was the first successful application of EVT over medical management,4 was presented and the RCT results were subsequently reported (Fig. 4A).5678 The results highlighted the importance of a small infarct core in patient selection and rapid in-hospital processes. The detailed information provided by these RCTs may have aided the patient selection process in period 2 of the current study. In reality, the importance of patient selection could be seen especially in late onset-to-puncture times. The probability of good outcomes in our study was reasonable even after 6 hours of onset-to-puncture time in the period 2 while this was steeply declined in the period 1.
Lastly, the procedure time was reduced and the rate of successful reperfusion increased. The development of newer devices with better performances likely explains these improvements in procedural outcomes. Additionally, the occurrence of SAH, which is usually related to vessel perforation during EVT, was less frequent in the later period. These factors may be related to the interventionists' experiences. Although treatment with stent retrieval is known to have less of a learning curve compared to other thrombectomy methods, the experience of interventionists also seems to be an important factor besides better and newer EVT devices. If interventional techniques can more overcome difficult cases,293031 the procedural outcomes would be better in the next round.
Our study has several limitations that should be considered when interpreting our results. Although the participating institutions consisted of comprehensive stroke centers in Korea, they may not appropriately represent all of the practices in Korea, since situations and preferences may differ according to each institution or interventionist. However, the baseline characteristics, reperfusion outcomes, and clinical outcomes of our study are similar to those in recent major RCTs,32 and our cohort outcomes show that the practice of EVT in Korea is continuously progressing. An additional limitation may be that our registry includes data from three independent center registries. As such, self-reports of clinical, procedural, and imaging information may have caused some inconsistent results. To minimize this limitation, the individuals who performed the core laboratory imaging analyses were blinded to the clinical results to ensure that the occlusion site, reperfusion grade, and safety outcomes were uniformly reported. Unfortunately, one of the most important variables, the mRS, should have been used by self-reports of each center owing to the retrospective nature of our study. Lastly, as this study is somewhat descriptive with a wide scope, more specific causes of the improvement, specific intervention for in-hospital care, and specified interventional techniques were not included in the current study. For the specific topics, further analyses and studies are planned to be done near future.
In conclusion, three Korean stroke centers have achieved comparable EVT outcomes to those observed in recently-reported major RCTs. Our real-world data show a wider range of EVT indications compared to those trials. Moreover, our study demonstrated that EVT outcomes have improved over time mainly owing to shortened procedure times, decreased hemorrhagic complication rates, and increased successful reperfusion rates. These factors seem to be associated with the improvement of in-hospital care systems and physicians' experiences in addition to the introduction of new and diverse devices (Fig. 4B).
Notes
This work was partly supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2014R1A5A2010008, S.I.S.).