The Combined Use of Intraluminal and Intrasaccular Flow Diversion for the Treatment of Intracranial Aneurysms: Report of 25 Cases
Article information
Abstract
Purpose
The Medina Embolic Device (MED) is a new intrasaccular device with promising early results. Previously we documented our initial experience of this device both alone and in combination with other devices including flow diverter stents (FDS). We sought to determine the effect of the MED + FDS strategy for the treatment of selected aneurysms.
Materials and Methods
We performed a retrospective analysis of prospectively collected data to identify all patients with aneurysms treated using both the MED and intraluminal FDS. We present our technical success rate, early and mid-term angiographic follow-up, and clinical outcome data.
Results
We identified 25 non-consecutive patients. The treatment was staged in 9 patients and in a single session 16 patients. The average age was 61±12.8 years (range 40–82). The average fundus height was 11±3.6 mm and average fundus width was 10.1±3.4 mm. In the staged cohort (n=9) at delayed angiography (mean 10 mths) 8 aneurysms (89%) showed complete exclusion (mRRC 1) and in one patient there was a parent vessel occlusion. In the simultaneous cohort delayed angiography (n=10, mean 8.1 months) demonstrated complete occlusion (mRRC 1) in 6 aneurysms (60%), 3 neck remnants (mRRC 2) (30%) and 1 patient (10%) showed persistent aneurysmal filling (mRRC 3a). There were 5 complications with permanent morbidity (mRS >2) in two patients. There were no mortalities.
Conclusion
The MED can be successfully used in combination with intraluminal FDS and in selected aneurysms this may represent an alternative to FDS and adjunctive coiling.
The Medina Embolic Device (MED; Covidien/eV3, Medtronic, Dublin, Ireland) is a new generation of intrasaccular flow diverter. The device has been granted CE mark in Europe and consists of a three-dimensional layered structure made from a radiopaque shape set core wire, and shape memory alloy filaments, which form a self-expanding mesh. Although there is limited experience of the device the available evidence suggests that it has a good safety profile and there is a negligible learning curve.123
In our previos publication we documented the use of the MED both alone and in conjunction with other devices such as standard intraluminal flow diverters.3 In this publication we documented the potential shortcomings of the MED when used as a stand-alone device however, we suggested that the device if used in combination with other devices may allow for extremely rapid occlusion of aneurysms, especially large aneurysms.
In this paper we document our experience on use of the MED with intraluminal flow diversion both sequentially and simultaneously using a jailing technique. We discuss the potential advantages of this technique and provide early follow-up on the use of both intraluminal and intrasaccular flow diversion in combination.
MATERIALS AND METHODS
Patient selection
We retrospectively searched our prospectively maintained database to identify patients treated with both the MED and an intraluminal flow diverter stent (FDS) between September 2015 and September 2017.
Endovascular treatment
We offered the use of MED to selected patients with unruptured aneurysms with a fundus diameter of ≥5 mm. The decision was based on individual aspects such as anticipated feasibility of this kind of treatment, propensity to coil compaction or aneurysm reperfusion. Patient informed consent was obtained before the procedure in all cases. All treatments were performed under general anaesthesia.
All patients received dual antiplatelet therapy (aspirin 100 mg daily and clopidogrel 75 mg daily) for at least five days prior to the treatment. The effectiveness of the antiplatelet regime was tested using the Multiplate analyser (Roche, Basel, Switzerland) and since 2016 the VerifyNow test (Accumetrics, San Diego, CA, USA) was also used. Patients found resistant to clopidogrel received 2×90 mg ticagrelor daily. The post-procedural antiplatelet regimen consisted of clopidogrel (or ticagrelor) continued for 12 months following treatment and aspirin continued for life.
Procedures were performed via the right common femoral route using a 6Fr access system as standard. For deployment of the MED a 0.021″ID Prowler Select Plus (Codman, Raynham, MA, USA) microcatheter was used. The framing MED was chosen based on a similar sizing method used for standard coils. In spherical saccular aneurysms with a fundus diameter of 9 mm or less the size of the first framing MED was purposely undersized by about 1 mm. After forming a spherical shape and adequate positioning of the framing MED it was mechanically detached and the requirement for further occlusion was assessed. Two commercially available intraluminal FDS were used: the Pipeline Embolisation Device (PED) (Covidien, Irvine, CA, USA) and p64 (phenox, Bochum, Germany). The selection of FDS was dependent upon the operators' judgement.
For deployment of the intraluminal flow diverter either a Marksman (Medtronic, Dublin, Ireland) catheter or an Excelsior XT27 (Stryker Neurovascular, Kalamazoo, MI, USA) catheter was used. In cases where a jailing technique was used the aneurysm was first catheterised followed by partial deployment of the intraluminal FDS to cover the neck. The MED was then deployed with the neck protection provided by the intraluminal FDS. After deployment of the MED the intraluminal FDS was fully deployed and detached.
All procedures were performed under heparin anticoagulation with a 5000 IU bolus dose at the start of the procedure and subsequent 1000 IU bolus doses every hour to maintain the activated clotting time between 2–2.5 times the baseline.
Procedural assessment and follow-up
Patency and flow characteristics within the aneurysm was assessed angiographically immediately after placement of the MED and the FDS and during follow-up. Procedural follow-up with digital subtraction angiography (DSA) is routinely performed initially at 3–6 months, again at 9–12 months and then once per year usually for three years or until the aneurysm was excluded from the circulation. In our early experience with the MED very early DSA follow-up was performed within the first two weeks.
Standard angiographic projections were used to assess the patency of the vessels and the aneurysms in addition to angiographic projections that repeated those used during the treatment. Aneurysm occlusion was graded using the modified Raymond-Roy classification (mRRC)4 or unchanged (patent).
RESULTS
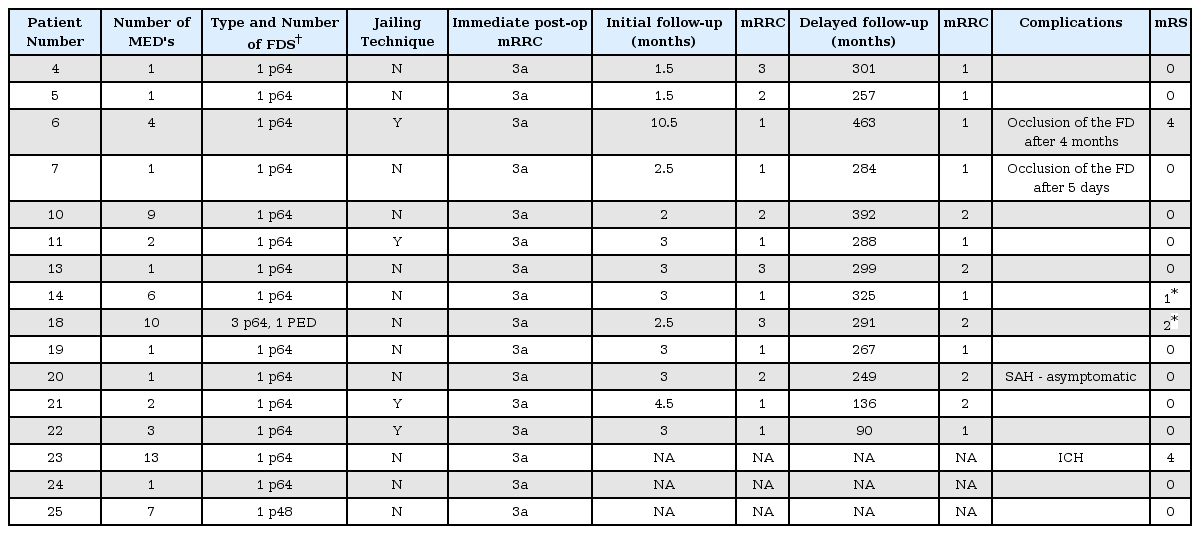
We identified 25 patients, 21 of which were female (84%) with 25 aneurysms that were treated with the MED and FDS. The average age of the patients was 61±12.8 years (range 40–82). Eleven aneurysms were located on the left, one aneurysm was in the midline the remaining 13 aneurysms were on the right.
Aneurysm location was the para-ophthalmic segment of the ICA (n=8), the cavernous ICA (n=4), the supraclinoidal segment (n=3), posterior communicating artery (n=5), ICA bifurcation (n=1), the M1 segment (n=1), the A1 segment (n=2), and anterior communicating artery (n=1).
The average dome height was 11±3.6 mm (range 5.9–17.2 mm), average dome width was 10.1±3.4 mm (range 6–17.5 mm) and average neck width was 5.6±1.8 mm (range 2–9.7 mm). The average aspect ratio was 2.1 and the average bottleneck ratio was 1.9. Three of the aneurysms presented with mass effect symptoms and the remaining were incidental. The aneurysm characteristics are summarised in Table 1.
Sequential Treatment
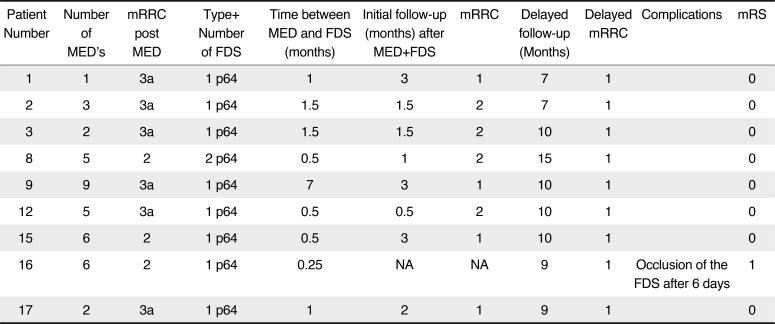
In total nine patients were treated sequentially with MED's placed in the aneurysm and then at a later date an intraluminal FDS was implanted (Fig. 1). An average of 4.3 (range 1–9) MED's were placed in each aneurysm and a single FDS was implanted to treat eight of the aneurysms. At the end of the MED implantation three patients had persistent neck remnants (mRRC 2) and the remaining six patients demonstrated persistent filling of the aneurysm sac (mRRC 3a). The FDS was implanted at an average of 1.6 months (range 0.25–7 months) after the MED's were implanted. Two intraluminal FDS were implanted in a single patient. The p64 FDS was used in all cases.
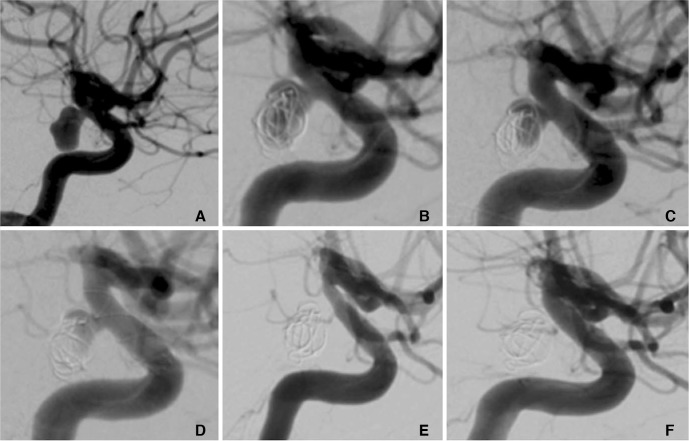
A patient in their 30's with an unruptured incidental right PComA aneurysm that measured 9×5 mm (A). At the initial treatment two MED's were placed in the aneurysm sac and at the end of the procedure sub-total opacification of the aneurysm was seen (B). At initial follow-up (one month) there was a significant neck remnant and filling of the proximal fundus (C). At this stage a single p64 FDS was implanted (D). Follow-up three months after implantation of the FDS showed complete exclusion of the aneurysm from the circulation (mRRC 1) and mild, asymptomatic, in-stent stenosis (E) that spontaneously resolved on delayed angiography (F).
At initial follow-up performed on average two months after implantation of the FDS (range 0.5–3 months) four aneurysms showed complete exclusion (mRRC 1), four showed neck remnants (mRRC 2) and one parent vessel occlusion had occurred. At the last follow-up performed on average 10 months (range 7–15 months) after the initial treatment eight aneurysms showed complete exclusion of the aneurysm. The results are summarised in Table 2.
Simultaneous Procedure including Jailing Technique
In total 16 patients the aneurysms were treated with MED and an intraluminal FDS during the same treatment session. In four cases a jailing technique (Fig. 2) was used and in 12 cases the MED's were placed and detached inside the aneurysm followed by deployment of the intraluminal FDS (Fig. 3). An average of 3.9 MED's were deployed (range 1–13) and an average of 1.2 intraluminal FDS were placed (range 1–4). The p64 was used alone in 14 cases, the p48 in one case and a PED was used in conjunction with the p64 in one case.
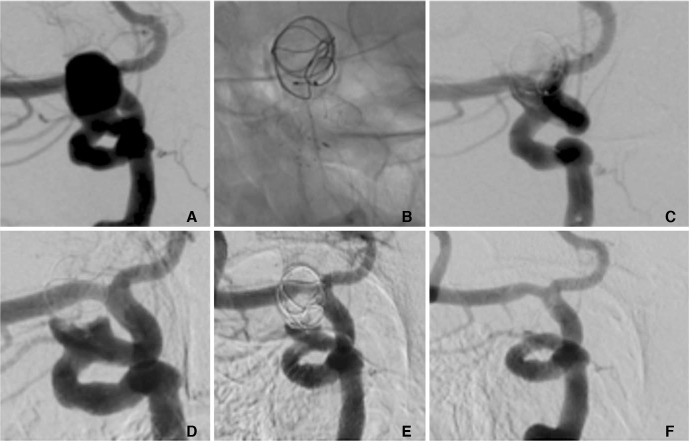
A patient in their 70's with an incidental 9×12 mm para-ophthalmic aneurysm (A) was treated initially with a MED with a p64 FDS deployed during the same procedure (B). At the end of the procedure there was persistent filling of the aneurysm (C). Early follow-up angiography (two months post-procedure) showed a persistent neck remnant (D), which gradually decreased over time (E). At delayed angiography (eight months) there is complete exclusion of the aneurysm from the circulation (mRRC I) (F).
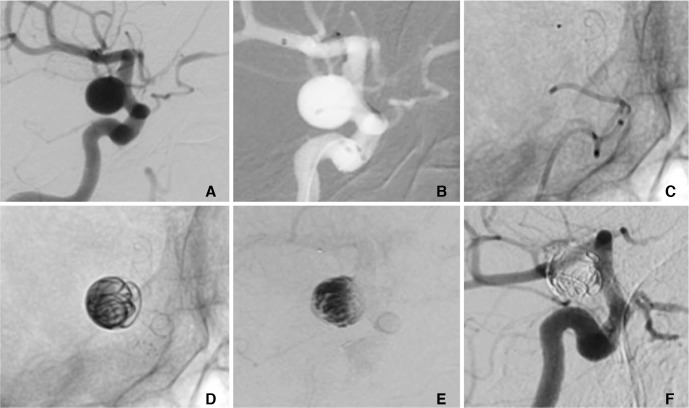
A patient in their 70's with an incidental 8×8 mm of the supraclinoid ICA (A) was treated with the MED and a p64 FDS using a jailing technique. After the catheterisation of the aneurysm and the M1 segment (B) the p64 was partially deployed until it completely covered the neck of the aneurysm (C). Subsequently, three MED's were deployed in the aneurysm (D) and the p64 was fully deployed and detached. Angiography at the end of the procedure showed marked contrast stagnation within the aneurysm (E). Angiography performed seven months post-procedure demonstrated complete exclusion of the aneurysm from the circulation (mRRC 1) (F).
Initial follow-up angiography was available for 13 aneurysms. One patient had an occlusion of the FDS at four months. Of the remaining 12 aneurysms angiography was performed on average at 2.8 months post-procedure, six (50%) aneurysms showed complete occlusion (mRRC 1), three aneurysms (25%) showed neck remnants (mRRC 2), and the remaining three patients (25%) showed persistent but subtotal filling of the aneurysm fundus (mRRC 3a).
Delayed angiographic follow-up was available in 10 patients and performed on average 8.1 months after the procedure at which point six aneurysms (60%) showed complete occlusion of the aneurysm (mRRC 1), three aneurysms showed neck remnants (30%) showed neck remnants (mRRC 2) and one patient (10%) showed persistent filling of the aneurysm fundus (mRRC 3a). The results are summarised in Table 3.
Complications
There were five complications, two of which led to permanent morbidity. There were no cases of mortality.
In three patients there was thrombosis within the FDS. In one patient this was asymptomatic (patient 16). Patient 6 developed an in-stent thrombus four months after the patient inadvertently stopped taking their prescribed anti-platelet medication. The patient was successfully treated with mechanical thrombectomy was left with permanent morbidity (mRS 4). In patient 7 in-stent thrombosis occurred five days post-procedure. The patient was successfully treated with mechanical thrombectomy and returned to baseline neurology (mRS 0). In one patient there was an asymptomatic sub-arachnoid haemorrhage, detected on routine post-operative imaging, the cause of which was unknown. In patient 23 after deployment of the MED, a MED coil loop protruded into the parent artery prior to the implantation of the FDS. The patient had been placed on dual antiplatelet medication prior to the operation however, a bolus dose of eptifibatide was given. Post-operatively there was a large intraparenchymal haemorrhage that was thought to be due to haemorrhagic conversion of an ischaemic lesion.
DISCUSSION
The MED represents a major advancement in the design of endosaccular flow diverters. In the first publication on the use of the MED, Turk et al.2 included nine patients, seven of whom were treated solely with the MED with aneurysms varying in size from 4.5 mm to 17 mm. Only three of the aneurysms had early follow-up at one month, all three showed >95% occlusion and the authors state that this limited follow-up provided an early indication that the device caused progressive aneurysm occlusion over time.
In our own publication on the use of the MED3 we presented the results of 15 consecutive patients with 16 aneurysms were reported. In this series 3.4 MED's per aneurysm were used (range 1–9). Adjunctive devices were used to treat several of the aneurysms. In this original publication we discussed in detail the potential advantages and disadvantages of the device including the problems regarding aneurysmal shape. The MED is designed to form a spherical shape however, many aneurysms are not spherical5 which could create a problem regarding the applicability of the device to many aneurysms. The device can and has been used in non-spherical aneurysms23 and can cause flow disruption however, the exact shape of the MED in the aneurysm may be difficult to assess. This difficulty in assessing the position of the leaflets of the MED within the aneurysm comes from the fact that these leaflets are almost radiolucent, even when using magnif ied exposures. Therefore, it can be difficult to determine the positioning of the leaflets particularly at the neck of the aneurysm. The most recent publication regarding the MED1 evaluated twelve patients with 13 aneurysms. In this series the authors used a single MED framer to obtain a basket in 12/13 aneurysms. They then proceeded to implant further MED fillers, standard coils or both. The authors state that, as this was their first experience with the device, even if total exclusion of the aneurysm was seen coils were used to fill the basket in order to prevent any retraction of the MED.
The introduction of intraluminal FDS represented a major advancement in the management of intracranial aneurysmal disease that allowed not only the occlusion of aneurysms but also the reconstruction of the parent artery. Intraluminal FDS are likely to promote the occlusion of aneurysms via a combination of effects. Initially intra-aneurysmal flow alteration promotes thrombosis however, the complete exclusion of the aneurysm from the circulation does not occur until after neointima has covered the neck of the aneurysm.678910 The intra-aneurysmal thrombosis will depend on the flow conditions created within the aneurysm after implantation of an intra-aneurysmal FDS. Aneurysm morphology, aneurysm location, neck size, angulation and many other factors may play a role in determining the inflow stream into the aneurysm. Similarly, the individual characteristics of flow diverters and the implantation strategy e.g. compressed, appropriately sized etc. will also play a role in the intra-aneurysmal haemodynamic flow post-operatively. Pereira et al.11 used dynamic DSA images to estimate the mean aneurysm flow velocity (mean aneurysm flow amplitude, MAFA) before and after FDS treatment. They demonstrated a larger decrease in mean aneurysm flow in patients that had occluded aneurysms at follow-up compared to those in whom the aneurysms remained patent. Other investigators have reported similar results of attenuated inflow and aneurysm occlusion1213 and in clinical cases the average reduction in intra-aneurysmal flow is in the region of 50–60%.141516171819202122 Although there have been no studies looking at the MAFA ration with intrasaccular flow diverting devices recent bench side studies have demonstrated that coverage at the neck is extremely important. Frölich and colleagues23 assessed the MED in aneurysm models. They showed that the degree of intra-aneurysmal flow disruption signif icantly correlated with the neck coverage (P=0.002) and the size of the neck (P=0.024). This data goes some way to confirming the clinical suspicion raised by others.2425
This process of aneurysm exclusion post FDS occurs gradually over time and the in the recent meta-analysis of Brinjikji et al.26 the complete aneurysm occlusion rate of aneurysms treated with intraluminal FDS was 76% at 6 months. The occlusion rate was slightly higher for small aneurysm (<10 mm) at 80% and 74% for large aneurysms (>10 mm). In a more recent meta-analysis of Zhou et al.27 that looked at the results of 59 studies and the treatment of 2263 patients with 2493 aneurysms a 6 month occlusion rate of 80.1% was documented that rose 90.8% at longer term follow-up (>12 months). Whilst these results are impressive approximately 10% of aneurysms will remain patent at 12 months and therefore, there is a potential risk posed by these aneurysms. Delayed aneurysm rupture post intraluminal FDS is well documented2829 and in a recent meta-analysis of 53 studies 81 cases of delayed aneurysms rupture were reported.30 The authors found that in cases when the rupture was documented the majority of cases or delayed rupture occurred within the first 1month after the treatment (77.58%). The majority of the delayed ruptured aneurysms, as would be expected, were located in the anterior circulation and the majority of the aneurysms were not giant aneurysms (53.7% of the aneurysms <25 mm). This result was similar to the results of the IntrePED study where 3/5 spontaneous ruptures occurred in giant aneurysms.31 The outcome in cases of delayed rupture was poor with just under 75% of delayed aneurysm ruptures resulting in death. The exact mechanism behind the delayed aneurysm rupture is incompletely understood. Some studies have demonstrated that intra-aneurysmal flow changes post FDS implantation may result in either an increase in intra-aneurysmal pressure32 or no signif icant decrease in pressure.21 Shobayashi et al.21 suggested that because of the persistent intra-aneurysmal pressure contrast stagnation or even the immediate disappearance of the aneurysm on post-treatment angiography does not necessarily mean that the aneurysm is protected against rupture. Other studies have suggested a potential role for intra-aneurysmal thrombus as a source for proteolytic enzymes that can break down the arterial wall and result in rupture.33 It has also been shown recently that flow conditions within the aneurysm lumen are associated with differences in aneurysm wall histology.34 It is not entirely inconceivable that based on the preoperative flow conditions certain aneurysms will not favour treatment with flow diversion alone due to the dramatic change in intra-aneurysmal flow induced by the FDS. However, this is yet to be ascertained.
In order to try and minimise the risk of delayed rupture, especially in large or giant aneurysms, concomitant coiling has been recommended by several authors.283536 Of note, 20% of the delayed ruptures in the meta-analysis of Rouchaud et al.30 occurred in aneurysms that were coiled. Unfortunately the packing density of the coiling was not recorded and therefore, further analysis is difficult however, it was suggested that a higher packing density might protect against rupture.
In order to quantify the different effects of FDS, coiling and FDS + Coiling Damiano et al.37 used finite element modelling and computational fluid dynamics (CFD) to compare the intra-aneurysmal haemodynamics of coiling, of various packing density, single FDS, multiple overlapping FDS, and FDS + coiling. This study showed that coils disrupt and impinge the inflow jet and that with increasing packing density (PD) there is decreased flow penetration into the aneurysm. A single FDS did not disrupt the vortex-like flow pattern but it does decrease the velocity of the inflow jet and of the impingement of the jet on the aneurysm wall. A single FDS accomplished a greater inflow rate reduction than coiling (PD<30%) however, coils at PD>30% results in a greater reduction in aneurysm-averaged inflow velocity than a single FDS. The adjunctive use of coils, upto a PD of 30%, in addition to a single FDS reduces average intra-aneurysmal velocity and wall shear stress (WSS) beyond that achieved by a single FDS alone. However, the addition of coils produced no further inflow rate reduction until the PD exceeded 11%. At low PD, <11%, the coils may act as a scaffold for intra-aneurysmal thrombus but they will have a limited haemodynamic effect. Similar additive effects of FDS and coiling have been seen by other groups.18 These studies may help to explain why even with adjunctive coiling some aneurysms rupture29 as well as the higher occlusion rate and lower risk of retreatment seen in patients treated with both FDS and coils compared to FDS alone38 since it is likely that a threshold of coil PD is necessary to achieve a meaningful clinical effect.
Although the work of Fröhlich23 did not compare the MED with standard coiling we believe that the flow disrupting effect of a single MED is likely to be significantly greater than that of a single coil. This is likely to reduce the inflow velocity as well as intra-aneurysmal flow characteristics that will protect the wall and promote thrombosis. Furthermore, the MED leaflets may provide a greater stabilisation of thrombus within an aneurysm as well as greater attenuation of the WSS due to their larger surface. We believe that this combination treatment may be particularly useful in partially thrombosed aneurysms. The flow redirection effect of the intraluminal FDS and the flow disrupting effect of the intrasaccular MED, along with the more even distribution of force afforded by the MED leaflets, may prevent the device being engulfed in the thrombus as may happen with coils.
Although in-stent thrombosis was seen in several of our cases we do not believe that there is an increase in the thromboembolic risk from this technique. In one of these cases this was caused by the patient inadvertently stopping their anti-platelet medications. In the other two cases the exact cause for the in-stent thrombosis was unknown although it is know that other medications can alter the effectiveness of anti-platelet agents.39 We test all patients for anti-platelet activity prior to the implantation of FDS however, it is impossible to monitor the response of patients post-operatively and we believe that it is imperative for the general physicians to be aware of possible drug interactions with the commonly used anti-platelet medications. Similarly, although more complications occurred in the ‘simultaneous’ treatment cohort we do not believe that this technique is inherently more dangerous. This technique is similar to stent assisted coiling which has been shown to have a similar safety profile to standard coiling40 however, larger studies are required to prove the technique is safe. As with wide necked aneurysms, jailing with the FDS may provide security and prevent the MED's from prolapsing into the parent vessel. However, it is possible that jailed microcatheter does not allow the intraluminal FDS to properly open and abut the arterial wall. This is a hypothetical risk and not something we have seen but we do believe it could occur and therefore, careful assessment of the FDS at the end of the procedure is required. When the aneurysm neck is relatively narrow, but the aneurysm is non-spherical, it may be more appropriate to deploy the MED's followed by the FDS but not use a jailing technique as it is not required.
We believe this is the f irst paper to look at the combination of intraluminal and intrasaccular flow diversion. Although these results are preliminary we believe that the additive effects of these two strategies may promote safe occlusion of aneurysms, particular large aneurysms that may otherwise carry inherently greater risk of rupture post FDS implantation. We believe aneurysms best suited to this treatment strategy include large aneurysms (>10 mm), multi-lobulated aneurysms and non-spherical aneurysms where there is a risk of a neck remnant after treatment with MED alone, and partially thrombosed aneurysms.
Our study has several limitations including those inherent to a retrospective design. In addition aneurysm location is varied as is the number of devices. In order to determine the effects of intraluminal and intrasaccular flow diversion on aneurysm filling and luminal flow we plan to perform bench side studies in the near future to evaluate the effects of MED and MED+FDS and hope to provide an indication as to the optimal number and combination of devices.
CONCLUSION
The use of intraluminal and intrasaccular flow diversion appears to be an effective treatment strategy for the treatment of aneurysms that can lead to rapid aneurysm exclusion. We believe that this may provide a useful alternative strategy in large and partially thrombosed aneurysms. Further studies are required to determine the usefulness of the techniques in ruptured aneurysms as well as the optimal combination of devices.
Notes
P. Bhogal and M. AlMatter serve as proctors and consultants for phenox.
H. Henkes is a co-founder and share-holder of phenox.
The other authors report no conflict of interest.