Embryological Consideration of Dural AVFs in Relation to the Neural Crest and the Mesoderm
Article information
Abstract
Intracranial and spinal dural arteriovenous fistulas (DAVFs) are vascular pathologies of the dural membrane with arteriovenous shunts. They are abnormal communications between arteries and veins or dural venous sinuses that sit between the two sheets of the dura mater. The dura propria faces the surface of brain, and the osteal dura faces the bone. The location of the shunt points is not distributed homogeneously on the surface of the dural membrane, but there are certain areas susceptible to DAVFs. The dura mater of the olfactory groove, falx cerebri, inferior sagittal sinus, tentorium cerebelli, and falx cerebelli, and the dura mater at the level of the spinal cord are composed only of dura propria, and these areas are derived from neural crest cells. The dura mater of the cavernous sinus, transverse sinus, sigmoid sinus, and anterior condylar confluence surrounding the hypoglossal canal are composed of both dura propria and osteal dura; this group is derived from mesoderm. Although the cause of this heterogeneity has not yet been determined, there are some specific characteristics and tendencies in terms of the embryological features. The possible reasons for the segmental susceptibility to DAVFs are summarized based on the embryology of the dura mater.
INTRODUCTION
Dural arteriovenous fistulas (DAVFs) are abnormal arteriovenous shunts that develop within an epidural venous space contained between the two layers of the dura mater; they are typically vascularized by meningeal arteries. Intracranial DAVFs account for 10–15% of all intracranial arteriovenous lesions, but no correlation has been observed between age and the frequency of aggressive neurologic symptoms [1-7].
The pathoetiology of DAVFs is unknown. However, there is a higher incidence in women of involvement of a certain anatomical region on the skull base and also a higher incidence in men of involvement of a specific topographical region [4,8,9]. There is a peak incidence between 30 and 50 years of age. For example, carotid cavernous DAVFs are often observed in the female population, and the shunt points of carotid cavernous DAVFs are mainly localized on the paramedian aspect of the posterior clinoid processes near the top of the clival bone. On the other hand, DAVFs located on the falx, olfactory groove, tentorium cerebelli, and lateral spinal cord are observed predominantly in the elderly male population, and this group always presents with cerebral or spinal venous hypertension and, consequently, impairment of the cerebral or spinal pial venous drainage, with aggressive clinical manifestations [6,8,10]. Even if it might be a small single fistula with a low-flow shunt, the severity of symptoms and the risk of hemorrhage are high in this elderly male population. Simply because the dural membranes of the falx, olfactory groove, tentorium cerebelli, and lateral spinal cord are apart from the main dural sinuses, 100% of the shunt flow drains directly into the leptomeningeal venous system. The meninx primitiva is a sort of meningeal mesenchyme formed from mesoderm (MS) or the neural crest (NC) [11-16]. Since the dura mater is formed from the meninx primitiva, the difference between MS and the NC can be a clue to understanding susceptibility to DAVFs and the heterogeneity of shunt locations.
MATERIAL AND METHODS
A consecutive case series of 66 DAVFs (32 men and 34 women; mean, 68.4 years) who have been performed catheter angiography was analyzed. Selective digital subtraction angiography and high-resolution cone beam computed tomography were performed in order to identify the shunt point. The topographical area derived from NC and MS was reviewed and identified from the literatures. And the relationship between germ layers and the distribution of the shunt point was defined.
ANATOMICAL CHARACTERISTICS OF DURAL MEMBRANE IN THE CENTRAL NERVOUS SYSTEM
Dura mater means a hard mother or tough mother in Latin. This dural membrane touching the bony structures is composed of two layers. One is the dura propria, which is the internal layer of the dura mater, and the other is osteal dura, which is also called periosteal dura [8,11,17].
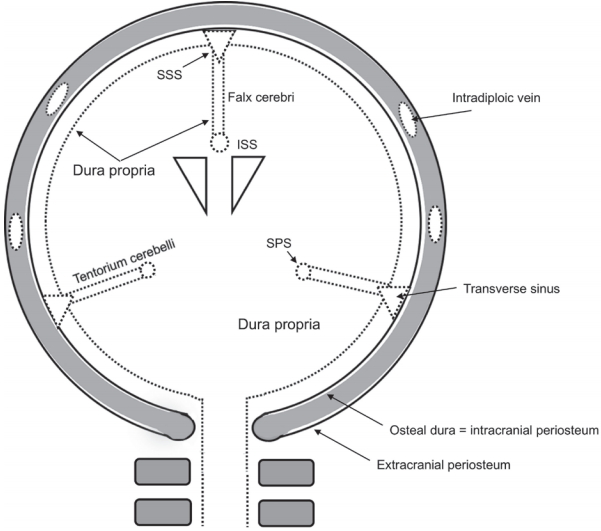
Dura propria is an internal layer of the dura mater which covers the neural tissue and subarachnoid space. Dura propria is a continuous fibrous sheath formed and shaped around the brain and spinal cord, its vessels, and its nerves during embryonic development. It is evident that the morphology of the cranial dura and that of cranial bones, both originating from the initial ectomeninx, as well as the disposition of the cerebral vessels and cranial nerves, are direct consequences of the development of the brain. The embryonic development of human frontal and temporal lobes involves the formation of the anterior and middle cranial fossae with the forward displacement of the eye and the orbit [8,12,18]. Another consequence in regard to the morphology of the dura mater is the formation around the brain of dural folds involving the dura propria. The falx cerebri is a dural fold formed between the bilateral cerebral hemispheres, and the tentorium cerebelli is a dural fold formed between the telencephalon, diencephalon and cerebellum (Fig. 1).

The topography of dura propria and osteal dura is shown. Note that the falx cerebri, tentorium cerebelli, and spinal dura are composed only of dura propria. These structures belong to the neural crest group. SSS, superior sagittal sinus; ISS, inferior sagittal sinus.
The dura mater of the falx cerebri, inferior sagittal sinus, tentorium cerebelli, and falx cerebelli and dura mater at the level of the spinal cord are composed only of dura propria which is derived from the NC cells [17,19,20]. The olfactory groove is considered as the most anterior part of the falx cerebri. In fact, the dural membrane on the midline of the olfactory groove continues to the rostral part of the falx in the microscopic observation [21]. These dura maters can be categorized to the NC group. Although the microstructure of dural membrane of the olfactory groove is the transitional part of the dura propria and osteal dura, crista galli is the most midline portion of the olfactory groove and it is frequently affected with the shunt of DAVF and composed mainly from dura propria. Therefore olfactory groove was defined as NC group.
The definition of superior sagittal sinus (SSS) is controversial. Because at the level of microarchitecture, the superior wall of SSS is considered as the osteal dura and the inferior surface is composed from the dura propria. This particular part might be transitional part between NC and MS. According to the experimental model by Jiang et al. [22] based on the transgenic mouse with a permanent NC cell lineage marker, SSS belongs to the NC origin. Therefore, SSS was categorized to the group of NC.
The topography of the NC group is characterized by the fact that they are a distance from the main dural sinuses except SSS, therefore, almost 100% of the shunt flow with DAVFs located on the NC group usually drains into the adjacent pial vein directly. Thus, the natural course of this group is usually aggressive and malignant. On the other hand, the major dural sinuses except the inferior sagittal sinus and the straight sinus are located beneath the bony structures of the skull, which means that they consist of both dura propria and osteal dura. These dura maters can be categorized as the osteal dura with dura propria group. This group corresponds to the MS because the dura mater in this group faces to the bony structure derived from the MS. The cavernous sinus, SSS, transverse sinus, sigmoid sinus, and anterior condylar confluence (ACC) at the level of the hypoglossal canal contact the surfaces of bony structures, and these dura maters belong to the MS group. The DAVFs located in the MS group are close and mostly on the surface of dural sinuses. This proximity allows the shunt flow to drain into the adjacent main dural sinuses. Therefore, the cortical venous reflux is not so significant unless there is thrombosis or a pathological steno-occlusive lesion in the affected dural sinuses. Concerning the microscopic localization of the shunt points, some of the MS group have specific shunt points, not on the surface of the dura maters, but in the bony structures [10,23]. For example, the terminal feeders of the cavernous DAVFs usually run in the bony structures of the upper clivus bone that corresponds to the posterior clinoid process. The dominant feeders of ACC DAVFs are usually intraosseous arteries running in the bony structure surrounding the anterior margin of the foramen magnum [24]. These terminal feeders are usually supplied from the branch of the contralateral ascending pharyngeal artery through the bony structure. Since the shunt points of the MS group are associated with endochondral bone and they often localize within the bony structure, these arteriovenous shunts should be called not dural AVFs, but transosseous or osteodural AVFs [23].
Several different fistula locations have been reported in patients with ACC, hypoglossal canal or anterior condylar vein, extracranial ACC, and within the bone. Discussions about the location of fistulas have continued to this day, with a lack of detailed anatomical studies concerning osseous venous structures in this region. Although ACC is an extracranial venous structure, the shunt point is usually not exactly inside of the ACC, but in the bony structure surround the hypoglossal canal. Recent study by Mizutani et al. showed also the shunt locates within the bony structures in the case of ACC DAVFs [25].
This intraforaminal space of hypoglossal canal contains both dura propria and osteal dura. The ACC DAVFs can be categorized to MS group, because the bone of this region is derived from the MS.
There are some transitional zones between the NC and MS groups. For example, the SSS, the confluence of the sinuses (torcular Herophili), and the boundary between the lateral surface of the tentorium cerebelli and the transverse sinus are defined as the borders between these two groups (Fig. 2).
THE NC AS THE EMBRYOLOGICAL FACTOR RELATED TO DAVFS
Although derived from ectoderm, the NC has sometimes been called the fourth germ layer because of its importance and pluripotency. The NCs are bilaterally paired strips of cells arising in the ectoderm at the margins of the neural tube. These cells migrate to many different locations and differentiate into many cell types within the embryo [15,26-28]. This means that many different systems (neural, skin, teeth, head, ectomeninx, face, heart, adrenal glands, and gastrointestinal tract) will also have a contribution from NC cells. During the 4th week post-conception, mesenchyme derived from the paraxial MS and NC condenses between the developing brain and foregut to form the base of the ectomeningeal capsule. This condensation heralds the earliest evidence of skull formation. During the late-somite period, the occipital sclerotomal mesenchyme concentrates around the notochord underlying the developing hindbrain. From this region, the mesenchymal concentration extends cranially, forming a floor of the brain [17,29,30]. Conversion of the ectomeninx mesenchyme into cartilage constitutes the beginning of the chondrocranium, starting at day 40 post-conception. The periosteum (osteal dura) of the frontal bones, which is of NC cell origin, contributes to the crista galli, falx cerebri, and the adjacent dura mater. Cells from the nasal septum are also derived from the NC and appear to further contribute to the falx cerebri. The anterior (rostral) prechordal region of the cranial base forms the future olfactory groove, sphenoid bone, frontal bone, and falx cerebri and is of NC origin [16,31-33].
During growth and development of the neurocranium, the dura mater regulates events in the underlying brain and overlying skull by the release of soluble factors and cellular activity. Morphogenesis of the cranial bones and sutures is dependent on tissue interactions with the dura mater, which control the size and shape of bones, as well as sutural patency [16,34,35].
DIVERSITY IN THE CLASSIFICATIONS OF DAVFS
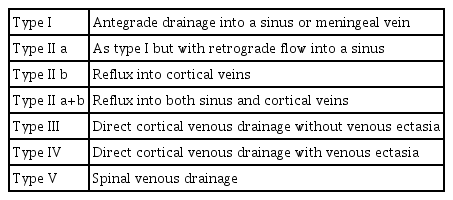
Several types of classifications or grading systems have been reported in the previous literature. Djindjian and Merland [36] first described the hemodynamic patterns of DAVFs based on the findings of cerebral angiography. They already noticed that the degree of cortical venous reflux was correlated closely with the risk of hemorrhage (Table 1). Lalwani et al. [37] reported that DAVFs were a hemodynamic disease that could progress at variable rates, from minimum venous restrictive disease associated with a low risk to more severe venous outflow obstruction associated with significant morbidity and death (Table 2). Cognard et al. [38] and Gomez et al. [39] modified Djindjian and Merland’s classification. According to this classification, the natural course of DAVFs can be predicted, and it thus provides a clear indication for intervention (Table 3). Borden et al. [40] proposed a simpler classification that focused on the flow condition of the affected sinuses and the existence of cortical venous reflux (Table 4). All these classifications were well designed from the standpoint of the hemodynamic condition of DAVFs, including the direction of the flow in the affected sinuses and the degree of cortical venous reflux. These classifications succeeded well in predicting the risk of hemorrhage and defining the indications for treatment [7,10,39].
Baltsavias et al. [41] reported a revised classification of DAVFs based on a detailed analysis of the angioarchitecture using three factors: directness of venous drainage (depending on the location of the shunt); exclusiveness of the pial venous drainage (related to venous outflow obstruction); and venous strain (as evidenced by venous congestion or venous ectasias) (Table 5). The advantage of this classification is to differentiate the dural sinus shunts or bridging vein shunts by the directness of venous drainage. This concept leads to eight different subtypes of DAVFs and may be better suited to identify which patients present with aggressive clinical symptoms, which ultimately will benefit patients by identifying those at risk.
Geibprasert et al. [9] proposed a new classification based on the craniospinal epidural venous anatomy (Table 6). The craniospinal epidural spaces can be categorized into three different compartments, the ventral epidural, dorsal epidural, and lateral epidural groups. Their classification is independent from the hemodynamic factors. They focused on the anatomical aspects related to their specific roles in drainage of the bone and central nervous system. This classification showed that there were close relationships between the locations of the epidural venous structures and the clinical characteristics of the disease.
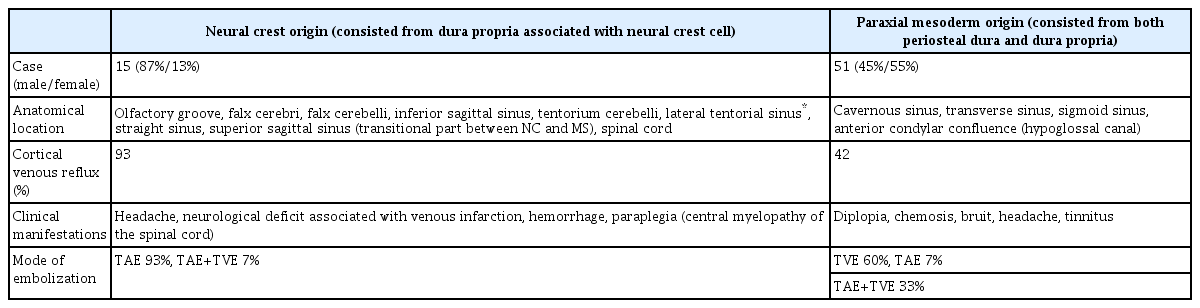
Tanaka [8] reported that the epidural space can be categorized into two types of domains (Table 7). One domain corresponds to the type of germ layers, that is NC and paraxial MS. The other domain corresponds to the type of embryological bony structures, that is endochondral bone and membranous bone. Additionally, this article showed that the dura mater of the olfactory groove, falx cerebri, tentorium cerebelli, falx cerebelli, and spinal cord consisted of only the dura propria, and this group was derived from the NC. This was one of the first articles suggesting that the type of germ layer may influence heterogeneity and the susceptibilities of the dura mater on the surface of brain and spinal cord in terms of the formation of DAVFs.
Regarding susceptibility to pathological calcification, Oyama et al. [42] reported a case of ectopic ossification in the cranial dura mater in a dialysis patient with secondary hyperparathyroidism. In this case, significant calcification was only noted at the tentorium cerebelli and falx cerebri, while the dura mater of the convexity was free from pathological calcification. Although the precise origin of the dural cells is obscure, they noted that dura mater develops from perineural tube mesenchymal tissue, which some NC-derived cells invade. Since NC cells are the origin of neurocranial bones, some of the cells in the dura mater have the potential to transform into osteoblast-like cells [12,15,30,34,43]. Based on such case reports and several basic studies with microscopic observation using immunohistochemical techniques, the dura mater mainly consisted from the dura propria (i.e., olfactory groove, falx cerebri, inferior sagittal sinus, tentorium cerebelli, falx cerebelli, and dura mater at the level of the spinal cord) is derived from the NC [22,44].
The dura mater consisting of both the osteal dura and the dura propria form the cavernous sinus, SSS, transverse sinus, sigmoid sinus, and ACC (hypoglossal canal). These structures are derived from paraxial MS. These two simple anatomical domains (NC and MS groups) clearly indicate the characteristics of the DAVFs in terms of the aggressiveness of the clinical course. The concept of the embryological domains related to these two germ layers is summarized in Table 8 based on the 66 consecutive cases of DAVFs seen in our institute over the last 10 years. The mode of embolization, either a transvenous or transarterial approach, can also be defined based on these two anatomical domains [4,5,8,45]. For DAVFs of the NC group, transarterial embolization is certainly indicated, simply because the NC group is separate from the main dural sinuses, and the transvenous approach is usually not indicated. Since DAVFs of the MS group are always associated with major dural sinuses, they can be primarily managed with the transvenous approach. Although there are several reports hypothesizing that DAVFs are an acquired disease associated with chronic venous hypertension or sinus thrombosis, these factors may play a role, not only as an initiator, but also as a promotor to induce the DAVFs. There are several controversial points which are not yet clarified. For example, the superior petrosal sinus is the part of lateral area of the tentorium cerebelli which might be the part of the NC, however, it is also in contact with the surface on the ridge of petrosal bone that is derived from MS. The dura mater of the sphenoid wing consists from both the dura propria and the osteal dura, however the clinical behavior of the sphenoid wing DAVFs is usually aggressive because of the proximity to the deep middle cerebral vein. In this way the categorization of the superior petrosal sinus and the sphenoid wing venous structure are still unknown and they might have two aspects of both the NC and the MS.
SUMMARY
From the standpoint of germ layers, there are only two types of domains in the dura mater. One is the dura mater from the NC, and the other is from paraxial MS. The DAVFs associated with the NC present with a higher incidence of significant venous reflux into the leptomeningeal venous system with malignant clinical manifestations in the elderly male population. The DAVFs on the surface of the dura mater derived from MS show less incidence of cortical venous reflux, with a relatively benign clinical course and female predominance [2]. The domains of dura mater based on the germ layers may affect their susceptibilities to the formation of DAVFs. The concept of the germ layers might be one of the clues to clarify the pathoetiology of DAVFs.
Acknowledgements
The author is grateful to Prof. Dae Chul Suh for suggesting the topic of this article.