Delayed Rupture of an Anterior Communicating Artery Pseudoaneurysm Caused by Distal Occlusion Thrombectomy Using a Stent Retriever: A Case Report and Mechanism of Injury
Article information
Abstract
We report a case of delayed rupture of an anterior communicating artery (Acom) pseudoaneurysm following mechanical thrombectomy (MT) of a distal artery occlusion using a stent retriever. An elderly patient with right hemiparesis showed left proximal internal cerebral artery and middle cerebral artery occlusions. During MT, a fragmented thrombus moved to the anterior cerebral artery (ACA). A stent retriever was deployed to the occluded ACA, and the Acom and proximal ACA segment were significantly straightened. Additionally, we attempted a blind exchange mini-pinning (BEMP) technique, but a subarachnoid hemorrhage (SAH) occurred. Bleeding was almost entirely absorbed 9 days after the procedure, but the SAH recurred at 20 days, and computed tomography angiography revealed a new pseudoaneurysm formation in the Acom. We suggest that the proposed mechanism of pseudoaneurysm formation was likely due to the dislocation and avulsion of the Acom perforators when the ipsilateral ACA was pushed and pulled during MT.
INTRODUCTION
Mechanical thrombectomy (MT) has been established as the primary treatment for large vessel occlusion in acute ischemic stroke (AIS). Indications for MT are gradually increasing, with the total number of MTs performed worldwide rising year by year [1]. One complication of MT is subarachnoid hemorrhage (SAH), which occurs in approximately 5.8% (4.5–7.2) of all MT cases; however, the incidence rate of SAH is known to be quite variable in the early stages (0–30%). Most SAH cases are asymptomatic (84%), and reports of rebleeding are rare [2].
The occurrence of a pseudoaneurysm has been reported in several post-MT cases with SAH [3-5]. Case reports exist for both methods of aspiration [3] and stent retriever [4,5]; however, the mechanism is not completely known. The reported locations of pseudoaneurysms are the middle cerebral artery (MCA) and its distal branches [3-5].
Here, we report a case of delayed rupture of an anterior communicating artery (Acom) pseudoaneurysm following distal occlusion thrombectomy using a stent retriever.
CASE REPORT
Clinical Presentation
An elderly patient was transferred to our hospital because of a sudden change in mental status and right hemiplegia, which had occurred 80 min previously. Initial non-contrast computed tomography (NCCT) showed no high-density lesions. After intravenous injection of tissue plasminogen activator (tPA), computed tomography angiography (CTA) showed left proximal internal carotid artery (ICA) and MCA occlusions, and the patient was transferred to our hospital for further management. The patient’s National Institutes of Health Stroke Scale (NIHSS) was 19 points. Atrial fibrillation was confirmed by electrocardiography. The patient was transferred to the angiography suite as MT was required for a subsequent perfusion study.
Intervention
After puncturing the right femoral artery, a 9-Fr sheath was inserted, and a 9-Fr balloon-guiding catheter (BGC) (Medtronic, Irvine, CA, USA) was catheterized to the left proximal ICA. Aspiration thrombectomy using a Penumbra pump (Penumbra, Alameda, CA, USA) was performed once using the BGC, and a large thrombus was observed. Subsequent angiography showed a recanalized ICA, an occluded MCA, and a newly occluded ipsilateral anterior cerebral artery (ACA) (Fig. 1A, C), which stemmed from distal migration of the fragmented thrombus. An 5 Fr intermediate catheter (Sofia, MicroVention, Aliso Viejo, CA, USA), Phenom 21 microcatheter (Medtronic), and Synchro-2 0.014-inch microwire (Stryker, Fremont, CA, USA) were used to navigate the left MCA and a Solitaire 4×40-mm stent retriever (Medtronic) was deployed in the occluded left MCA M1 segment. The Solumbra technique was performed twice [6]. The left MCA was fully recanalized to thrombolysis in cerebral infarction (TICI) grade III (Fig. 1B).
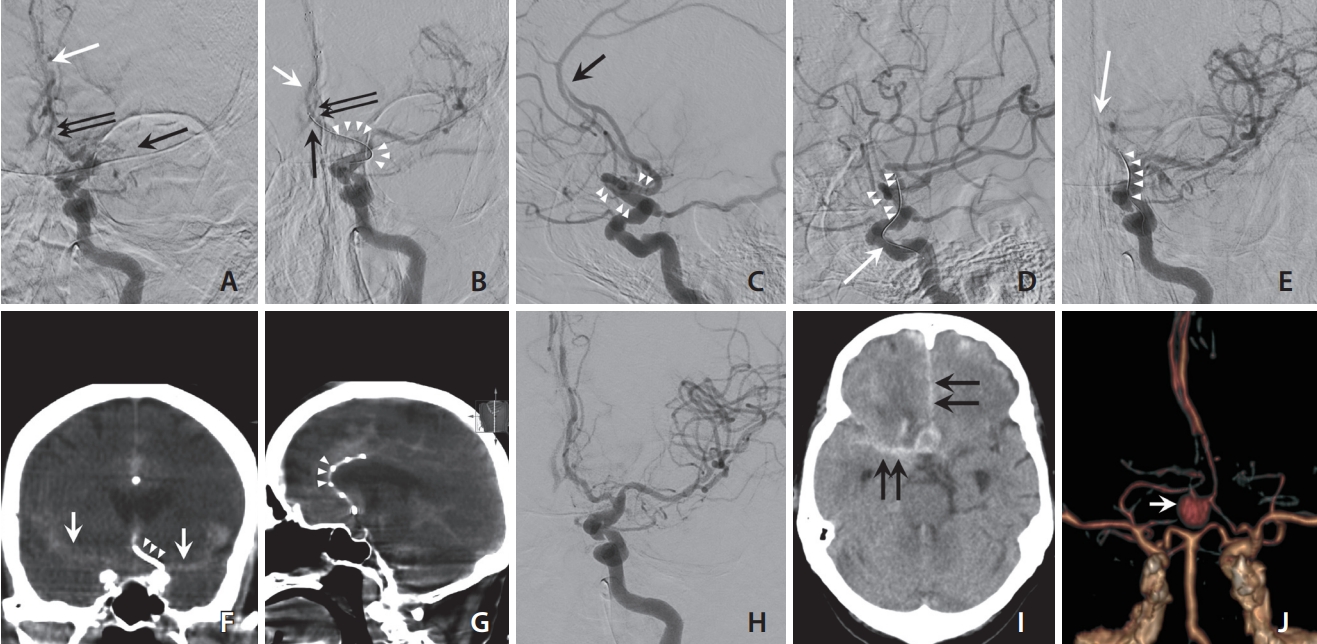
Angiographies and follow-up brain computed tomography angiography (CTA) of the patient. (A) After aspiration thrombectomy using a Penumbra pump in the left proximal internal cerebral artery (ICA), the left ICA angiography shows left middle cerebral artery (MCA) M1 and anterior cerebral artery (ACA) (arrows) occlusion. We performed the Solumbra technique (using Solitaire stent retriever [4×40 mm] and 5-Fr SOFIA intermediate catheter) in the left MCA M1 segment and the MCA was fully recanalized. (B) After deploying the stent retriever (Solitaire 4×40 mm, white arrow) to the distal ACA occlusion site, the blind exchange mini-pinning (BEMP) technique was tried. The left ICA angiography shows a heavily dislocated left ACA A2 segment to the medial side (white arrow), and the change in angle between distal ICA and ACA A1 segment (arrowheads). The anterior communicating artery (Acom) was also dislocated distally (black arrow). Note the change in angles between ACA A1 and A2 segments (double black arrows) before (A) and after (B) deploying the stent retriever. (C, D) Comparing lateral projection of left ICA angiographies before (C) and after (D) stent deployment in the left ACA occlusion site (black arrow), ipsilateral distal ICA and ACA A1 segments were also straightened (arrowheads). White arrow in (D) indicates the push wire of the stent retriever. (E) Working projection of left ICA angiography while removing the microcatheter over the push wire of the stent retriever shows that the angle formed by the push wire suddenly straightened (arrowheads), with no antegrade flow in the Acom and ipsilateral ACA (white arrow). (F, G) Subarachnoid hemorrhage (SAH) was confirmed by a flat panel computed tomography. The white arrows indicate SAH in bilateral interhemispheric and the Sylvian cisterns, and arrowheads indicate the push wire (F) and the stent strut of Solitaire stent retriever (G). (H) Final angiography shows fully recanalized state of left MCA and ACA. There was no visible aneurysm in Acom. (I) On the 20th day, SAH in the interhemispheric and right Sylvian cistern (black arrows) were observed again. (J) On that day, Acom pseudoaneurysm (white arrow) was observed on CTA.
While accessing the left ACA occlusion site, the microwire moved to the right ACA once through the Acom. After navigation of the left ACA, a Solitaire 4×40-mm stent retriever was deployed at the occlusion site. Because of severe tortuosity and the acute angle between the distal ICA and ACA A1 segments, the cerebral artery was significantly straightened when the stent retriever was deployed (Fig. 1C, D). In addition, the angle between the Acom and ACA A2 segment changed from acute to obtuse (78° to 136°) as the ACA was dislocated distally (Fig. 1A, B). Additionally, the blind exchange mini-pinning (BEMP) technique was utilized for fast recanalization in a single step [7-9]. While removing the microcatheter over the push wire of the stent retriever, the angle formed by the push wire suddenly straightened in this case. Immediately after angiography, blood flow in the Acom and ACA was not observed (Fig. 1E). Cone-beam computed tomography with a flat-panel detector was performed, and SAH was confirmed in bilateral interhemispheric and the Sylvian cisterns (Fig. 1F, G). After confirming SAH, attempts at the BEMP technique were stopped in the middle, and flow arrest was performed for 5 minutes by ballooning the BGC. Subsequently, the stent retriever in the ACA was resheathed and removed. Recanalization of the left MCA and ACA was confirmed on follow-up angiography (Fig. 1H), and the procedure was terminated.
Postinterventional Course
Immediate NCCT revealed a massive SAH; however, the patient’s neurological status did not deteriorate. We controlled the blood pressure carefully and closely observed for neurological deterioration. Nine days after MT, NCCT confirmed that nearly no SAH had persisted. We decided to start anticoagulation with oral apixaban 2.5 mg twice daily. Follow-up NCCT on the 14th day showed a high-density lesion in the anterior part of the suprasellar cistern. Since the clinical findings improved, we did not perform a closer examination of the cistern. The patient’s mental status suddenly deteriorated on the 20th day, and CTA showed a new SAH and Acom pseudoaneurysm (Fig. 1I, J). This wide-neck pseudoaneurysm was 7.5 mm in size (neck diameter, 4.8 mm) with anteroinferior projection.
Considering that a pseudoaneurysm had occurred after MT, we prepared a coil embolization procedure. However, the patient’s family members refused treatment, and the patient died 3 days later.
DISCUSSION
To the best of our knowledge, this is the first report of Acom pseudoaneurysm formation after MT in a patient with AIS. We hypothesized that there were 2 main causes of pseudoaneurysm formation. The first probable cause is damage to the Acom perforators resulting from the straightening and distal dislocation of the Acom (Fig. 2A, B). Severe tortuosity in cerebral vessels appears to be the main cause of dislocation. Another factor for dislocation seems to be the radial force of the stent retriever. Solitaire stents are known to have a relatively large radial force compared to other stents [10]. In this condition, more force is applied because the force to retrieve the stent retriever is difficult to transmit, which increases the likelihood of damage to the Acom perforators [5]. We also performed the BEMP technique to remove the microcatheter and exchange the aspiration catheter over the stent retriever’s push wire [7-9]. During this process, it is highly likely that the Acom perforators underwent excessive push and pull (Fig. 2C). Even in the exchanging microcatheter technique used in other interventional procedures, the tension stored in the exchange guidewire could become very large, and the control of the guidewire sometimes proves to be difficult [10]. The exchanging microcatheter technique is known to have a high risk of distal wire perforation [11]. The second probable cause is subintimal dissection of the Acom perforators by the microwire. Koge et al. [12] previously reported that intimal injury occurred due to the use of a large-sized stent device for a small-sized blood vessel. Several types of Acom perforators are known, and estimating from the location, the hypothalamic and chiasmatic branches seemed to be damaged in this case [13].
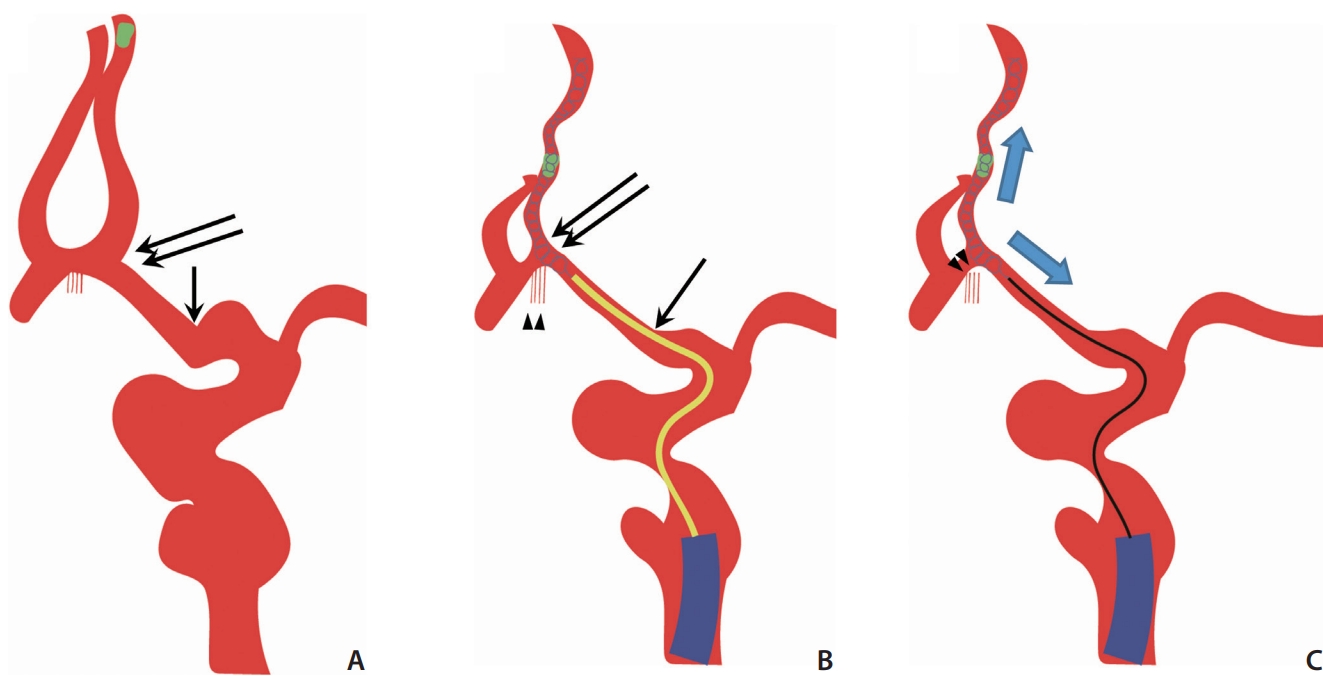
A proposed mechanism of anterior communicating artery (Acom) pseudoaneurysm formation. (A, B) The angle between the left anterior cerebral artery (ACA) A1 and A2 (double black arrow), and the angle in proximal ACA A1 segment (black arrow) were changed after deploying the stent retriever, and Acom perforators also dislocated distally (arrowheads). (B, C) When attempting the blind exchange mini-pinning (BEMP) technique, microcatheter (yellow catheter) removal was attempted, and the stent retriever was pulled and pushed a lot (blue arrows) along the push wire. Eventually, Acom perforators were ruptured (arrowheads).
To date, pseudoaneurysm formation after MT has been reported in 3 cases [3-5]; 2 following stent retriever usage [4,5] and 1 following the aspiration method [3]. The explained mechanisms were direct injury for the aspiration method [3] and avulsion of small vessels for the stent retriever method [5]. Misaki et al. [5] reported that small arterial vessels were pulled proximally as the stent retriever was pulled. All 4 cases, including ours, occurred immediately post-MT SAH. Conversely, in the other 3 cases, focal SAH occurred near the occlusion site, which were the MCA M2 segments [4,5] and the distal M1 segment [3]. The time when pseudoaneurysm was confirmed varied from 8 hours [5], 4 days [4], and 15 days [3]. In our case, since there was no follow-up image, the timing of pseudoaneurysm occurrence was unknown.
Recently, MT has been carefully implemented even for medium vessel occlusion (MeVO) [7-9]. Among the various thrombectomy methods for MeVO, the BEMP technique showed a low symptomatic intracranial hemorrhage (ICH) rate. This came from an increased first-pass recanalization rate by performing aspiration and stent retriever methods simultaneously [8]. The BEMP technique has been reported mainly for MCA M2 occlusions [7,9], and 1 study reported it being used for ACA and posterior cerebral artery branch occlusions [8]. In our case, we attempted it in a distal ACA occlusion and used a heavier device (Solitaire 4×40 mm) than what was used those in previous reports (Trevo 3×20 mm [7], Aperio 3.5×28 mm [8], Catch mini 3×15/3×20 mm [8], Tron 2×15/4×20 mm [9]). Since the use of thrombectomy for MeVO is expected to increase in the future, it should be considered that the BEMP technique might increase the incidence of hemorrhagic complications in some circumstances.
Distally located vessel occlusions, higher number of thrombectomy device passes, and intravenous tPA use are known risk factors for the occurrence of post-MT SAH [2]. This could result in devastating but avoidable complications. Therefore, in distal vessel thrombectomy, an option may be to analyze the advantages and disadvantages of distal vessel thrombectomy. In our center, when SAH occurs after MT, NCCT is usually performed serially rather than angiography. Therefore, the possibility of pseudoaneurysm formation may have been overlooked under these circumstances. Since post-MT SAH is not rare, we suggest that every post-MT SAH case should undergo follow-up CTA in a timely manner. Since apixaban may have accelerated the rebleeding, administration should be considered after follow-up CTA, confirming the absence of a bleeding source.
In conclusion, we report a case of delayed rupture of an Acom pseudoaneurysm after distal occlusion thrombectomy using a stent retriever. In AIS cases of distal tortuous vessel occlusion, it is necessary to decide the implementation of MT more carefully. If MT is performed in these circumstances, a smaller device may reduce the symptomatic ICH rate. Furthermore, it is necessary to pay attention to whether the vessels undergoing the procedure are straightened. If straightening is significant, it may be necessary to avoid an additional procedure. Finally, if SAH occurs as a complication of MT, follow-up CTA should be conducted in a timely manner.
Notes
Fund
This work was supported by a National Research Foundation (NRF) grant (2020R1I1A3067073) funded by the Korean government.
Ethics Statement
This study was approved by the local Institutional Review Board, and the board waived the need for patient consent. Informed consent for publication was not obtained so we anonymized the patient.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: D-HS and SWY. Analysis and interpretation: D-HS and SWY. Data collection: D-HS. Writing the article: D-HS. Critical revision of the article: SWY, YD, and JKD. Final approval of the article: SWY. Obtained funding: SWY. Overall responsibility: SWY.
