Reversible Symptom Aggravation by Intake of Taurine-Rich Foods in Patients with Venous Congestive Myelopathy: Controlled Case Series Study
Article information
Abstract
Purpose
Reversible aggravation of myelopathy symptoms was observed after the intake of taurine-rich foods in patients with venous congestive myelopathy (VCM) caused by a spinal arteriovenous shunt (SAVS), and the taurine-challenge test was applied to demonstrate an association between taurine and VCM.
Materials and Methods
The current study reviewed any aggravation history of myelopathy symptoms, including walking difficulty, after consuming taurine-rich foods among 133 consecutive patients with a SAVS from a prospective institutional database from June 2013 to February 2021. The type of taurine-rich foods, demographic data, arteriovenous shunt level, and follow-up periods were obtained. For the controlled taurine challenge test, Bacchus® (Dong-A Pharmaceutical, Seoul, Korea), a taurine-rich drink, was given to patients who fulfilled test criteria of recovered VCM (pain-sensory-motor-sphincter scale ≥2, improvement of spinal cord signal intensity on magnetic resonance imaging, and follow-up >6 months after SAVS treatment) to confirm the disappearance of such aggravation.
Results
Ten patients had an aggravation history related to food. Webfoot octopus, small octopus, squid, crab, scallop, and taurine-rich energy drink (Bacchus®) were related to such aggravation in patients with VCM. Aggravation appeared about 30 minutes after food intake followed by expressions such as ‘I could not walk and collapsed to the ground’ and usually lasted for about 3 hours, followed by a slow recovery after taking rest. Four patients who met the test criteria underwent the taurine challenge with Bacchus® and revealed no further symptom aggravation, suggesting that taurine did not affect patients after recovery from VCM.
Conclusion
The association between taurine-rich food and reversible symptom aggravation can appear in patients with VCM and disappear after VCM treatment. Aggravation of venous hypertension in the spinal cord is suggested as a mechanism but further elucidation is needed.
INTRODUCTION
Neurological symptom aggravation after steroid medication is observed in at least 38% of patients with venous congestive myelopathy (VCM) and often results from impaired venous outflow secondary to a spinal arteriovenous shunt (SAVS) [1]. Some patients may have symptom aggravation, especially patients with VCM, although steroid medication is empirically suitable for myelopathy induced by various causes, including spinal cord trauma, transverse myelitis, and cord tumor. Evaluation of steroid effects is often neglected probably due to vague positive expectations of steroid effects in cord edema reduction and patient conditions that are difficult to evaluate in the current neurological examination system, especially in patients with VCM [2]. Besides steroids, no known medication or food aggravates VCM induced by SAVS. One of the typical symptoms of VCM includes aggravation of VCM-associated symptoms after vigorous excise or during afternoon hours [3,4].
Taurine (2-aminoethanesulfonic acid) is a stimulant commonly found in energy drinks that purportedly demonstrates a positive association with sports performance, although several studies have not reported any such association [3]. It is the most prevalent free amino acid found in mammalian muscle tissue and is present in high concentrations in meat and seafood [5]. Taurine corresponds to 50–60% of free amino acids in mammals and fulfills some essential biological functions. Within muscle fiber, taurine has exhibited positive effects on athletic performance in animal models. A randomized study observed a 16% increase in fat oxidation during a cycle ergometer test at a moderate intensity for 90 min in trained cyclists [6].
The current study observed that some VCM patients showed transient and reversible aggravation of neurological symptoms after the intake of taurine-rich food or drinks, and the disappearance of such aggravation after recovery from VCM was demonstrated using the taurine challenge test. The current study discussed the mechanism of how taurine can affect the blood-spinal cord barrier.
MATERIALS AND METHODS
During the management of patients with a SAVS, we found that some patients with VCM caused by a SAVS had a history of aggravation after eating raw fish or some seafood. It was hypothesized that those symptom aggravations were associated with certain seafood like web-octopus as well as certain energy drinks that contained taurine. The aggravation appeared about 30 minutes after intake of the food and was associated with expressions like “I could not walk and collapsed to the ground”. Aggravation usually lasted for about 3 hours, and then patients slowly recovered after taking rest. Some patients repeatedly experienced these events, and the symptom aggravation was so surprising that they feared the situation and avoided the food afterward. Thereafter, we reviewed the presence of such history related with any food retrospectively as well as prospectively. After reviewing the history, taurine was suspected as a causative component because the seafood that the patients ate was also known to be rich in taurine [5]. Such an association with taurine was finally demonstrated in the selected study patients who met the test criteria by the taurine challenge test, which was designed to reveal that taurine did not affect patients after recovery from VCM.
For 133 patients with a SAVS from June 2013 to February 2021 in a prospectively-collected institutional database, all patient history before and after treatment was obtained and reviewed. Items reviewed in patient history included the presence of any symptom aggravation associated with any food, what kind of food, how much was eaten, any symptom patterns, and the time course regarding aggravation and recovery.
According to the literature, signs and symptoms of vascular myelopathy as in VCM usually start insidiously and progress gradually with a variable degree of gait instability/imbalance, stiffness or clumsiness in the extremities, weakness in the arms or legs, urinary urgency or hesitancy, bowel incontinence, and the phenomenon of a sensory level [7-9]. This study defined myelopathy as having the presence of sensory, motor, or sphincter dysfunction as evaluated by the pain-sensory-motor-sphincter (PSMS) score and abnormal T2 signal intensity in the spinal cord on magnetic resonance imaging (MRI) [2]. A high-resolution spinal angiography was obtained to confirm a SAVS in these patients. Those patients with a SAVS underwent endovascular embolization to treat the shunt. The procedural details for treatment have been previously described [10-16]. Regarding the symptom aggravation, detailed symptom progression and recovery were recorded from patients who clearly remembered the event because it surprised them. After experiencing this aggravation, often the food was avoided due to the painful memories. Patient neurological status was evaluated by the PSMS score [2]. Pre- and post-treatment PSMS were compared. Study permissions from the Institutional Review Board (IRB no. 2021-0891) and informed consent from each patient for the taurine challenge test and publication were obtained.
Taurine Challenge Test after Treatment
The current study conducted a taurine challenge test with an energy drink, Bacchus® (Dong-A Pharmaceutical, Seoul, Korea), that contains 2,000 mg of taurine to determine any association between taurine and VCM. Candidates for the taurine challenge test were selected from patients with follow-up >6 months who showed neurological symptom improvement (≥2 in total PSMS score) after treatment (Fig. 1). Patients without follow-up for >6 months, without improvement (≥2 in total PSMS score), or with high signal intensities in the spinal cord on sagittal T2-weighted images (suggesting residual VCM after treatment) were excluded (Fig. 2). Finally, 4 patients met the selection criteria and took the taurine challenge test (Table 1). The patients had a variable degree of psychotic trauma regarding taurine-rich food or drinks and were given the choice to take the test at home (n=3) or at the hospital (n=1). Informed consent was obtained with the assurance of safety and reversibility in case of symptom aggravation development. After drinking a bottle of Bacchus® (Dong-A Pharmaceutical) containing 2,000 mg taurine, candidates were observed at for least 3 hours under the guidance of a neurologist. The patient who took the test at home was monitored by telephone for any symptom changes.
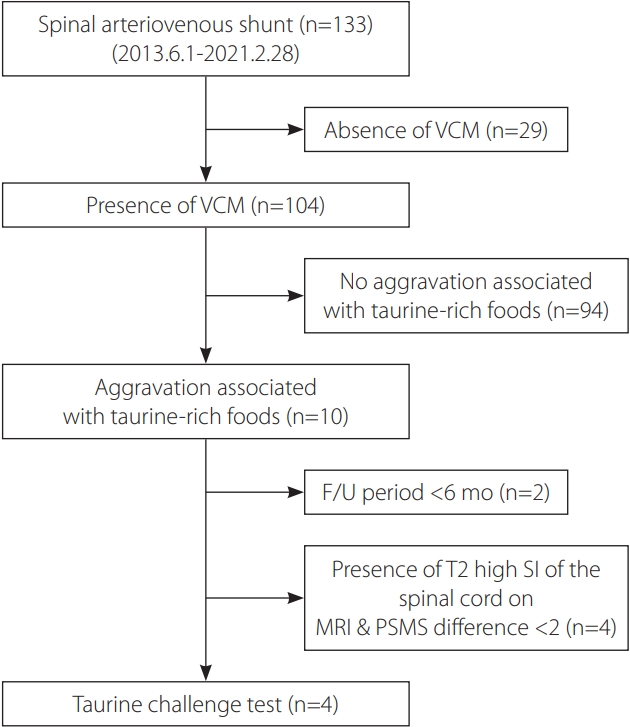
Candidate selection process diagram for the taurine challenge test. VCM, venous congestive myelopathy; F/U, follow-up; SI, signal intensity; MRI, magnetic resonance imaging; PSMS, pain-sensory-motor-sphincter score.
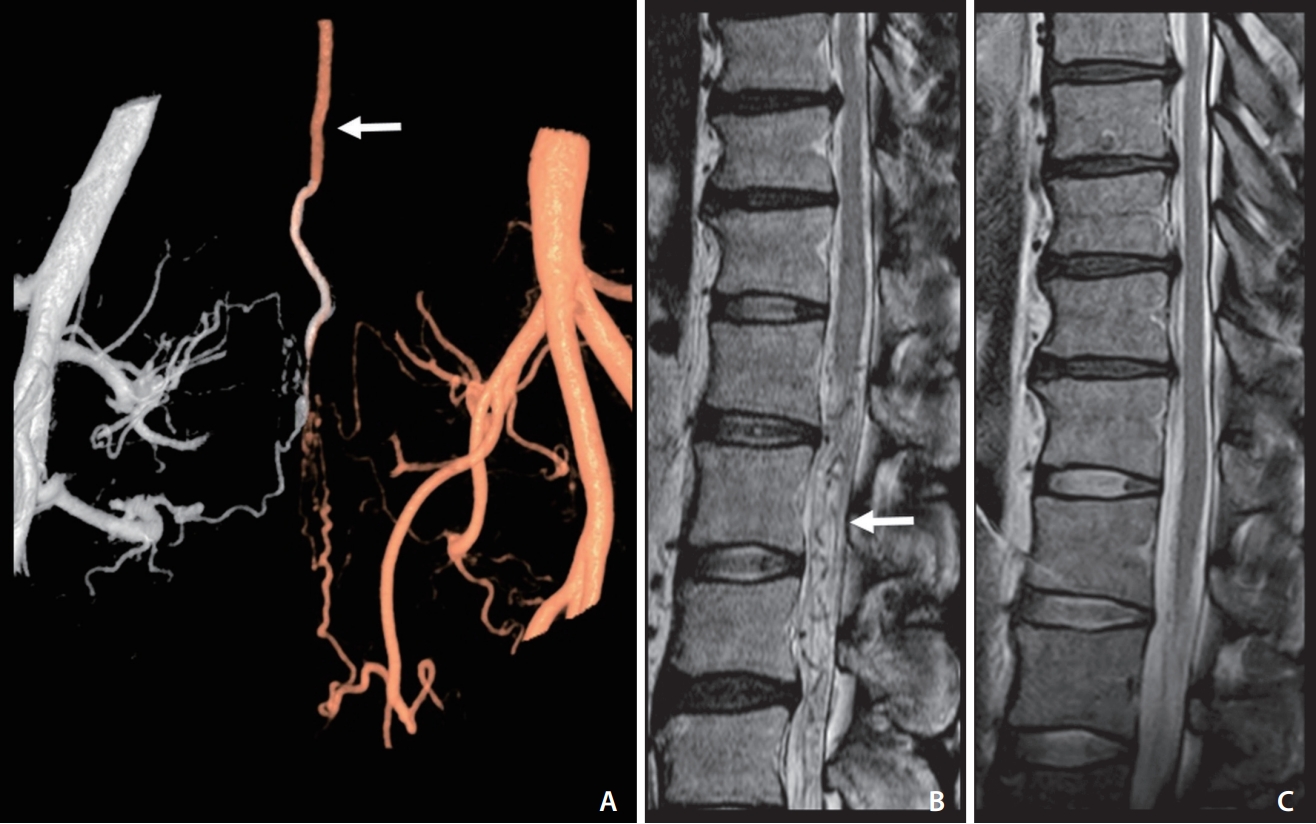
An adult patient with reversible aggravation myelopathy symptoms that developed after intake of taurine-rich foods and disappeared after treatment. (A) A fusion 3D angiogram of both internal iliac arteries shows a sacral dural arteriovenous shunt with retrograde regurgitation via the radicular vein (arrow). (B) Venous congestive myelopathy was noted in the spinal cord on a T2-weighted image in magnetic resonance imaging (MRI). Note the signal voids of the vessels around and below the swollen spinal cord (arrow). (C) Follow-up MRI after shunt treatment shows the disappearance of high signal intensities on the spinal cord and also revealed no symptom development after the intake of taurine-rich food.
RESULTS
The current study identified 10 patients who had a history of transient symptom aggravation after consuming food or drink. Patients with symptoms associated with food were detected in patients with VCM (Fig. 1). The food included webfoot octopus, squid, scallops, and crab. The taurine-rich drink was Bacchus® (Dong-A Pharmaceutical), a famous tonic drink in Korea, which contains 1,000 and 2,000 mg of taurine in Bacchus® F and Bacchus® D, respectively.
Symptom aggravation typically starts approximately 30 minutes after the ingestion of food or drink and is confined to the lower extremities in which preexisting weakness or walking difficulty developed. The patients could not walk, could not go up the stairs, and required being seated for hours due to neurological symptom aggravation. The symptoms existed for 2–3 hours and then slowly improved to the previous status. An uncomfortable feeling sometimes remained in the lower extremities for several hours after symptom duration.
Taurine Challenge Test
The taurine challenge test revealed no symptom aggravation in 4 patients, and thus was regarded as recovery from VCM. After a negative test result, patients tried taurine-rich food without the development of any symptoms. Until being asked to take the taurine challenge test and retry taurine-rich food, the patients had not eaten their favorite taurine-rich food due to previous bad memories. A patient (case 1) who revealed no response to the taurine challenge test showed numbness in both arms and legs without any motor deficit after ingesting 500 mg of webfoot octopus (estimated taurine content was approximately 7,000 mg) by self-cooking. The arms recovered first and then the legs. This response was not regarded as a positive result of the taurine challenge test because it was a subtle numbness without motor deficit that included walking difficulty and no arm symptoms and was initially noticed apart from the disease. Such numbness may be related to the intake of a large amount of taurine-rich food.
DISCUSSION
VCM symptoms caused by a SAVS can be aggravated after steroid medication [1]. However, it is believed that the current study is the first report showing that taurine-rich food or drink can also aggravate such myelopathy symptoms in patients with VCM caused by a SAVS. The taurine challenge test revealed taurine did not affect patients after recovery from VCM. In addition, intake of taurine-rich food after a negative taurine challenge test did not show any further symptom development after free intake of taurine-rich food. Therefore, the current study concluded that a clear association exists between the intake of taurine-rich food and transient reversible symptom aggravation in patients with VCM caused by a SAVS. The frequency and degree of symptom aggravation according to the type of taurine-rich foods were also suggested by some patients to be related to taurine concentration in the food or drink [5].
The symptom degree and duration caused by intake of taurine-rich food or drink (approximately 3 hours) were shorter than those of steroid medication (>12 hours), especially with intravenous steroids or pulse therapy with methylprednisolone [1]. Although such differences in degree and duration were not completely elucidated, it seemed to be caused by the high dose of intake or intravenous steroid injection [1]. Aggravated symptoms included paraplegic (bilateral) paralysis in the lower extremities in which the symptom preexisted from the onset and made walking impossible. The event was traumatizing for patients who remembered the situation very clearly and avoided taurine-rich food thereafter.
Webfoot octopus, squid, crab, and scallops were the taurine-rich food that patients ingested. The taurine contents of the aforementioned seafood were 1,306, 358, 317, and 222 mg/100 g, respectively [5]. Webfoot octopus, which had the highest taurine content among the kinds of seafood, is a common favorite dish, and franchise restaurants serving webfoot octopus are popular in Korea. A typical amount served for 1 person is 150 g webfoot octopus, which corresponds to approximately 2,000 mg of taurine, similar to an energy drink like Bacchus® D (Dong-A Pharmaceutical). Many different energy drinks contain vasoactive metabolites that are usually a combination of caffeine, taurine, glucuronolactone, and sugars [17]. Whether steroids or alcohol can also be regarded as having a restorative effect is questionable.
The transient symptom aggravation mechanism associated with taurine is still unclear. VCM is caused by venous hypertension to the spinal cord by shunt flow regurgitation along the radicular vein. Such transvenous hypertension results in vasogenic edema, which affects plasma water movement into the perivenous vascular space and then the extracellular space of the spinal cord parenchyma by increased hydrostatic pressure induced by venous hypertension (Fig. 3) [1]. Such vasogenic edema seems to occur without crossing the blood–spinal cord barrier (Aquaporin-4) via the peri-arterial space, and plasma water crosses via the peri-venous space in the spinal cord instead [18,19]. Enhancement on MRI in VCM may also suggest the possibility of a contrast agent entering the extracellular space via a damaged Aquarin-4 water channel in the perivenous space [20-22]. Considering that vigorous exercise can aggravate symptoms in patients with VCM, taurine-rich food may be similar to such exercise-induced energy loss in the neuronal cells of the spinal cord, leading to transient symptom aggravation [23,24].
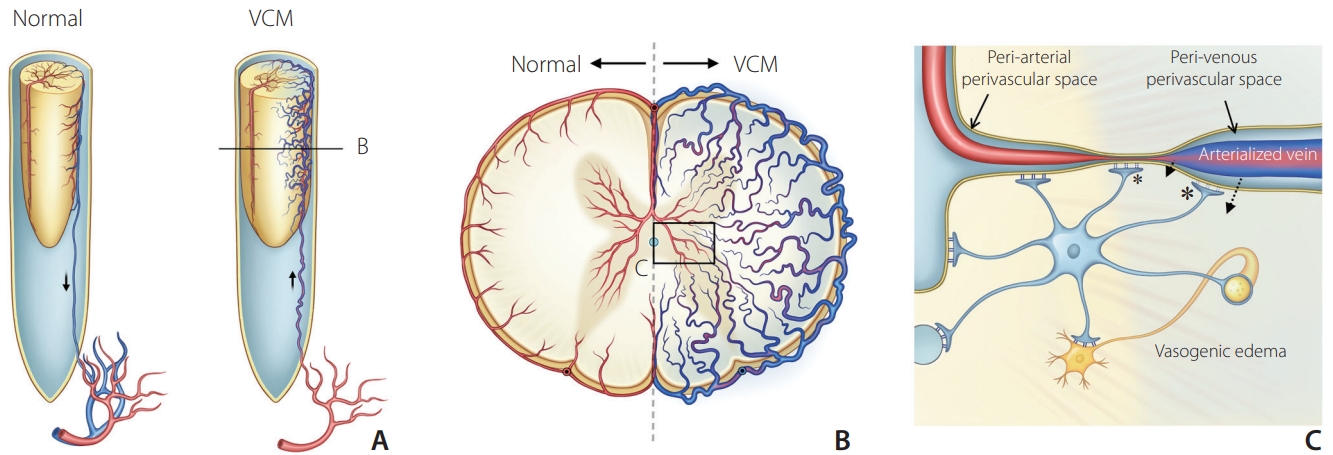
Schematic diagram of possible mechanism related to the vasogenic edema in venous congestive myelopathy (VCM). (A) Arterialized venous engorgement around the spinal cord and cord swelling in the spinal dural arteriovenous shunt. (B) Cross-section of the swollen spinal cord showed venous engorgement of arterialized veins with decreased arterial perfusion in the VCM. (C) Magnified view of the vasogenic edema shows increased hydrostatic pressure caused by venous hypertension that opens Aquaporin-4 water channels in the foot processes of astrocytes and induces perivascular water movement (dotted arrows). Damage of blood-spinal cord barrier at the venous perivascular space (large asterisk) and capillary wall (small asterisk) finally increases interstitial fluid in the extracellular space leading to vasogenic edema.
CONCLUSION
Reversible symptom aggravation can occur after intake of taurine-rich food in some patients with VCM related to a SAVS. The taurine challenge test revealed taurine did not affect patients after recovery from VCM. Patients afterward freely ingested taurine-rich food similar to before symptom development. Therefore, the current study suggests that the intake of taurine-rich food is associated with transient reversible symptom aggravation in some patients with VCM, that patient history may help in the diagnosis of vascular myelopathy, and that avoidance of such food intake during VCM management may be recommended.
Notes
Fund
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2018R1A2B6003143). The authors have no relevant financial or non-financial interests to disclose.
Ethics Statement
Study permissions from the Institutional Review Board (IRB no. 2021-0891) and informed consent from each patient for the taurine challenge test and publication were obtained.
Conflicts of Interest
DCS has been the Editor-in-Chief of the Neurointervention since 2018. No potential conflict of interest relevant to this article was reported. YS has been the Assistant Editor of the Neurointervention since 2019. No potential conflict of interest relevant to this article was reported. No other authors have any conflict of interest to disclose.
Author Contributions
Concept and design: DCS. Analysis and interpretation, data collection: SJ, YHC, SMC, SYY, AYS, YML, BK, YS, and DCS. Critical revision of the article: SJ, YML, BK, and YS. Writing the article, final approval of the article, obtained funding, overall responsibility: DCS.

