Double-Balloon-Assisted Coiling for Wide-Necked Posterior Communicating Artery Aneurysms with a Fetal-Type Variant of the Posterior Cerebral Artery: A Case Series
Article information
Abstract
Endovascular treatment for wide-necked posterior communicating artery (PcomA) aneurysms with a fetal-type variant of the posterior cerebral artery (PCA) is often challenging. Since the complete occlusion rates achieved with the currently available treatment methods are unsatisfactory, we aimed to study the effectiveness of a double-balloon-assisted technique for these aneurysms. From September 2014 to August 2020, 6 consecutive patients with PcomA aneurysms with fetal-type PCAs and no previous treatment were treated with this technique at our institution (3 ruptured cases and 3 unruptured cases). The indication for this technique is that the neck of the aneurysm should significantly and broadly incorporate both the internal carotid artery and fetal-type PCA, such that a single-balloon remodeling and single stent would be inadequate to protect both the arteries. In all patients, the fetal-type PCAs were preserved without a stent and with adequate occlusion status. This double-balloon technique can be effective in the treatment of these aneurysms.
INTRODUCTION
Endovascular treatment for widenecked posterior communicating artery (PcomA) aneurysms with a fetal-type variant of the posterior cerebral artery (PCA) is often challenging. This is because these aneurysms tend to incorporate the origin of the fetal-type PCA and internal carotid artery (ICA). Excessive obliteration can lead to PcomA sacrifice with ischemic complications [1,2]. Moreover, incomplete occlusion to preserve the PcomA can lead to recanalization because PcomA aneurysms are one of the representative aneurysm locations with a high likelihood of recanalization [3]. Several types of endovascular treatment have been reported for these aneurysms, including the double-microcatheter technique, single balloon-assisted technique (advancing a balloon microcatheter into the ICA or PcomA), and single or Y-configuration stent-assisted technique [1,4]. Additionally, flow diversion with a Pipeline embolization device (Medtronic, Minneapolis, MN, USA) is currently used for such aneurysms. However, some recent studies have reported the use of this device to be less effective in occluding wide-necked PcomA aneurysms with fetal-type PCA [5,6].
Herein, we describe a double-balloon-assisted coiling technique for wide-necked PcomA aneurysms with fetal-type PCA. This technique enables good neck remodeling to protect the ICA and PcomA and possibly avoid the use of a stent-assisted technique.
CASE SERIES
From September 2014 to August 2020, 6 consecutive patients who had PcomA aneurysms with fetal-type PCAs and no previous treatment for these aneurysms were treated with double-balloon-assisted coil embolization at our institution. Our inclusion criterion for double-balloon-assisted coiling was as follows: the neck of the aneurysm should significantly and broadly incorporate the ICA and fetal-type PCA, such that a single-balloon remodeling and single stent would be inadequate to protect both arteries. The clinical and radiological features, procedure details, and angiographic and clinical outcomes were reviewed retrospectively.
This retrospective study was conducted in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments and was approved by our institutional review board. The requirement for informed consent from patients was waived owing to the retrospective study design.
Technique Details
All endovascular treatments were performed under general anesthesia. All patients received an intravenous bolus of 3,000 to 5,000 units of heparin just after a puncture, followed by an intravenous infusion of 500 to 1,000 units per hour for the duration of the procedure to ensure the activated coagulation time was maintained at 2 to 3 times the baseline level. In patients with unruptured aneurysms, aspirin (100 mg/day) and clopidogrel (75 mg/day) were administered for 2 weeks before the treatment. In a ruptured aneurysm, 1 antiplatelet drug was started after the procedure. Fig. 1 shows details of the double-balloon remodeling (case 1 in Table 1). Through a femoral site, a 7-French (Fr), 80-cm Cook shuttle sheath (Cook Medical, Bloomington, IN, USA) and a 7-Fr, 100-cm Fubuki guiding catheter (Asahi Intecc Co., Ltd., Aichi, Japan) were placed at the ICA cervical portion. Two 4×11-mm Scepter XC balloon microcatheters (MicroVention Inc., Aliso Viejo, CA, USA) were introduced into the PcomA and ICA. In this procedure at the PcomA side, a microguidewire was advanced distally up to PCA P2 segment to relieve a ledge effect and introduce the Scepter into the PcomA. In the process, sufficient care should be taken because the advancement of the microguidewire and balloon catheter into the PcomA and PCA can cause stretching of the vessels, which might damage the perforator. An Echelon 14 microcatheter with a 45° angle (EV3, Irvine, CA, USA) was placed within the aneurysm sac. Coiling was performed with inflation of both Scepter catheters. One of the most important tips was how to use the 2 balloon catheters. Neck remodeling using the 2 balloon catheters with inflation was performed when the first coil was inserted for flaming the aneurysm. Considerable caution was paid, especially when the balloon at the PcomA was inflated because excessive inflation of the balloon catheter could lead to fatal vessel rupture. Just before the detachment of the first coil, whether the coil protruded into the ICA or PcomA was confirmed with deflation of the 2 balloon catheters. After that, at the stage of inserting the filling coils and finishing coils, the 2 balloon catheters were mainly used in case the following coils were just about to be placed outside the flaming coils, or the microcatheter for coiling was just about to be pulled back from the aneurysm. Repeated inflations and deflations of the 2 balloon catheters were conducted with care to set the interrupting time within 5 consecutive minutes to prevent thromboembolic complications. After coiling, only a microguidewire at the PcomA was left when the Scepter catheter at the PcomA was pulled back. This is because pulling back the Scepter relieves the stretch of the PcomA. In this procedure, whether additional stent assistance is needed to prevent coil protrusion to the origin of the PcomA or ICA should be examined.
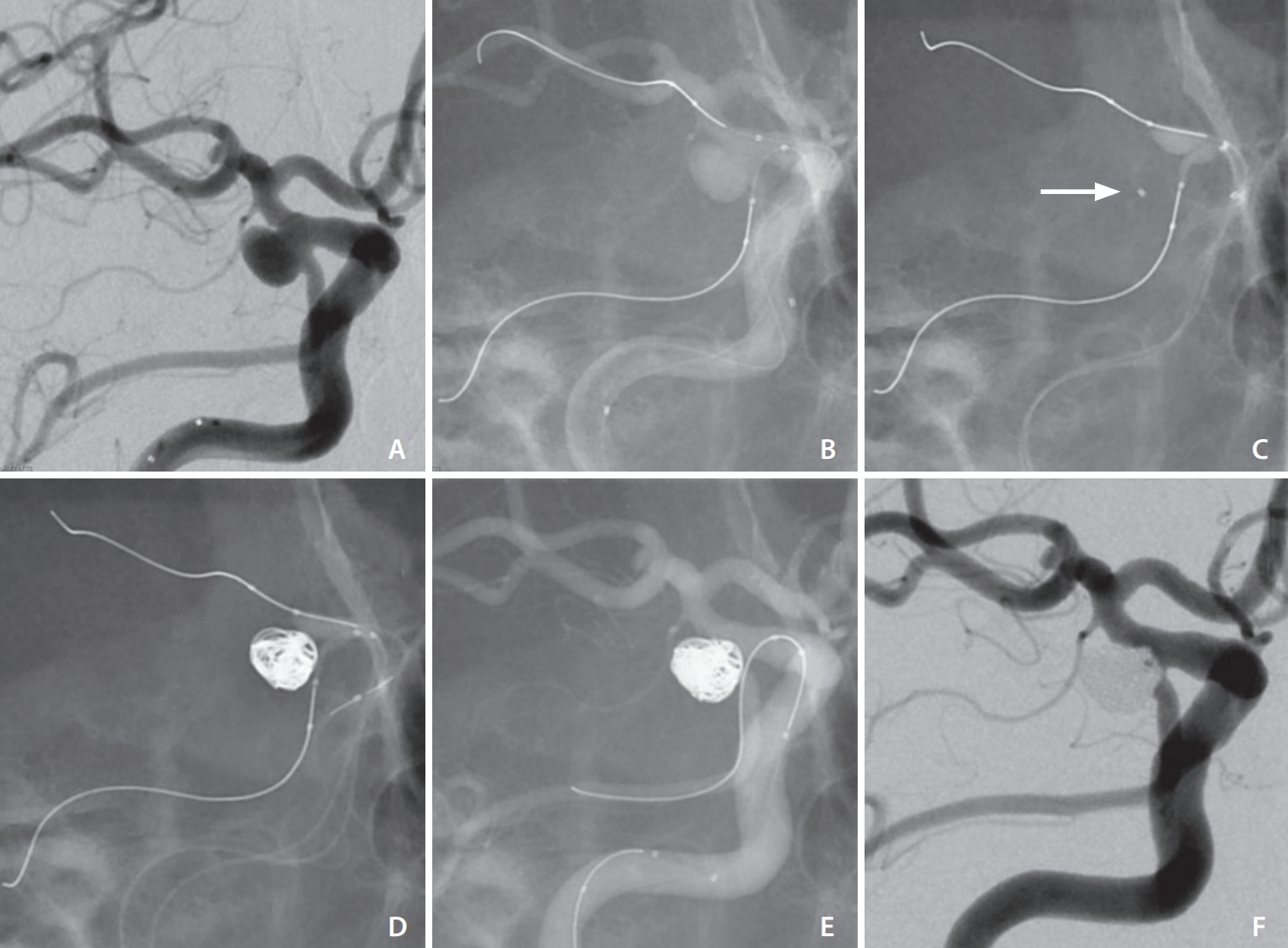
Double-balloon-assisted coiling for right posterior communicating artery (PcomA) aneurysm with fetal-type posterior cerebral artery (PCA). (A) The aneurysm incorporates the internal carotid artery and fetal PCA. (B, C) Two Scepter XC catheters and a microcatheter for coiling (white arrow) are introduced. (D) Coiling is performed using 2 microballoon-catheters. (E) Only a microguidewire at the PcomA is left to examine stent placement at the PcomA. (F) Postprocedural angiogram showing adequate occlusion with PcomA patency.
Clinical characteristics of 6 patients with PcomA aneurysms with fetal-type PCAs treated with double-balloon-assisted coil embolization
In addition, there are some clinical situations where the angle of the PcomA originating from the ICA is acute, making it difficult for the microguidewire for the Scepter catheter to advance towards the PcomA. Even in that situation, our technique can be applied. One Scepter catheter can be inflated at the ICA distal to the origin of the PcomA, which can change the direction of the microguidewire into the PcomA. In addition, the Scepter located at ICA with inflation can facilitate the advancement of the other Scepter into the PcomA even after the microguidewire proceeded distally to the PCA (Fig. 2, case 2 in Table 1).

Double-balloon-assisted coiling for right posterior communicating artery (PcomA) aneurysm with fetal-type posterior cerebral artery with PcomA acutely originating from the internal carotid artery (ICA). (A) Right ICA angiogram shows the difficulty in direct advancement of a microguidewire towards the PcomA. (B) A Scepter is placed at the ICA distal to the PcomA origin. (C) The Scepter distal to the origin of the PcomA with inflation facilitates access of the microguidewire into the PcomA for the other Scepter. (D) Two Scepter XC catheters and a microcatheter for coiling (white arrow) are prepared. (E) The first coil was inserted and the protrusion of the coil into the parent arteries was checked with deflation of the 2 balloon catheters. (F) Complete occlusion with PcomA patency.
Results
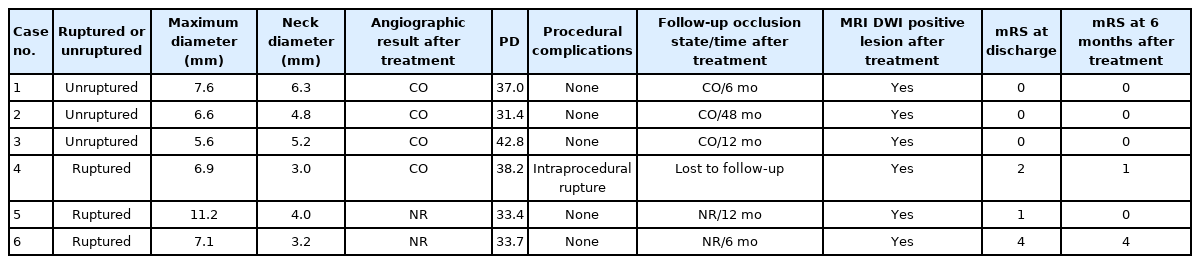
The 6 eligible patients were aged 55 to 79 years and 5 of them were female. There were 3 cases each of unruptured aneurysms and ruptured aneurysms. The maximum size of the aneurysms ranged from 5.6 mm to 11.2 mm. In all patients, fetal-type PCAs were preserved without stent use and with an adequate occlusion status (4 complete occlusion cases and 2 neck remnant cases). One case of a procedure-related complication, i.e., intraprocedural rupture, occurred. This intraprocedural rupture was noted during coil placement when there was a protrusion outside the aneurysm. However, hemostasis using the 2 Scepter catheters was achieved without morbidity. Table 1 shows the results of all the cases. Follow-up with magnetic resonance angiography or digital subtraction angiography (≥6 months) was conducted in 5 patients who maintained a stable occlusion status. Only 1 patient (case 4 in Table 1) was not followed-up in imaging, as the patient did not visit our hospital after being transferred to a rehabilitation hospital. All figures of the other cases except case 1 and 2 in Table 1 are described in the Supplementary Data (Supplementary Figs. 1–4).
DISCUSSION
This case series describes the usefulness of the double-balloon-assisted coiling technique for wide-necked PcomA aneurysms with fetal-type PCAs.
A fetal-type PCA is marked by a small-caliber P1 segment relative to the PcomA [7]. The estimated prevalence rates of unilateral and bilateral fetal-type PCAs are 26% and 4%, respectively [8]. Thiarawat et al. [9] reported that the occurrence of PcomA aneurysms was associated with a higher prevalence of ipsilateral fetal-type PCAs in terms of hemodynamic flow.
Regarding the history of the double-balloon remodeling technique, Takahashi [10] reported the technique for a basilar tip aneurysm for the first time, using 2 Grapevine-10 balloon catheters (Medtronic MIS, Sunnyvale, CA, USA, and Fas-Stealth; Target Therapeutics, Fremont, CA, USA). Spelle et al. [11] popularized the technique using HyperGlide balloons (EV3, Plymouth, MN, USA). A technique with the double-lumen balloon catheters, including Scepter XC, has also been reported [12]. To date, double-balloon remodeling has been introduced mainly for bifurcation-type aneurysms, and the results and tips of the technique for PcomA aneurysms with fetal-type PCAs have rarely been reported.
Chen et al. [1] have reported cases of PcomA aneurysms with fetal-type PCAs treated with the double-microcatheter and single-balloon techniques located at the PCA. In the double-microcatheter technique, there are possibilities of more inadequate embolization or technical failure in severely wide-necked aneurysms together with delayed hemostasis due to the absence of balloon microcatheters when intraprocedural aneurysmal rupture occurs. In the single-balloon technique, inflation of only 1 balloon microcatheter to protect both vessels may result in overinflation, which can lead to fatal vessel rupture. Compared with the aforementioned techniques, double-balloon-assisted coiling has several advantages. First, the use of 2 balloon microcatheters at the ICA and PcomA enables firm and safe neck remodeling with more complete embolization and easy intraoperative temporary flow arrest in case of intraoperative rupture (case 4 in Table 1). Second, it can also decrease the necessity of stent use. This is important, especially in ruptured cases, because in cases with acute rupture, sufficient antithrombotic therapy before stenting is not provided compared with that in unruptured cases. Third, using the balloon-assisted technique enables high packing density [13]. In our case series, to decrease recanalization in the long term, the target first packing density and total volume packing density were set as >15% and >30%, respectively [14].
Double-stent-assisted techniques, such as the kissing-Y-stent technique and λ stenting technique, are reported as alternative methods [1,15]. Specifically, in unruptured PcomA aneurysms with fetal-type PCAs, the stent-assisted technique, including double-stent-use may be positively examined because sufficient antithrombotic therapy can be initiated before stenting. However, these techniques are complex, and the incidence of thromboembolic complications may increase in insufficient antithrombotic conditions [16]. Even if stent assistance is needed in the double-balloon-assist strategy when the balloons are deflated and a coil herniates into the parent arteries, low-profile stents (LVIS junior [MicroVention Inc.] or Neuroform Atlas [Stryker Neurovascular, Fremont, CA, USA]) can be deployed from the Scepter catheter as bailout technique [17]. In all our 6 presented cases, diffusion-weighted magnetic resonance image (DWI) performed on the day after the treatment showed scattered ischemic lesions, although they were asymptomatic. This may be related to the fact that the double-balloon-assisted coiling required 3 microcatheters. Takigawa et al. [18] reported that the increased number of microcatheters was significantly associated with thromboembolic events detected on DWI, although the symptomatic ischemic rates did not significantly increase. Thus, we may evaluate the platelet reactivity and adjust the antiplatelet agents, if necessary, especially in unruptured cases.
Regarding the use of a flow diverter, several studies have reported unsatisfactory complete occlusion rates for PcomA aneurysms coupled with fetal-type PCAs at 0–66.7% [5,6,19]. Ten Brinck et al. [19] reported that a flow diverter for PcomA aneurysms with fetal-type PCAs seemed to be less effective than that for the other types of PcomA aneurysms, and alternative strategies should be considered as a first-line treatment. In addition, a microcatheter cannot be navigated into PcomA aneurysms through the mesh of a flow-diverting stent once the stent is deployed over the neck of the aneurysm. This means that there will be no effective endovascular treatment method if PcomA aneurysms with fetal-type PCAs are treated using a flow diverter and an adequate occlusion state is not achieved. Thus, the double-balloon-assisted coiling technique may help improve endovascular surgery for PcomA aneurysms with fetal-type PCAs.
Our study has some limitations. First, this study included only a small number of patients and had a short-term follow-up. Murakami et al. [20] reported that aneurysms in which recanalization was not observed within 2 years of endovascular coil embolization were stable over the course of a mean follow-up of 7 years. The Kaplan–Meier analysis of the non-recanalization rate showed that recanalization was mostly confirmed within 12 months of treatment [20]. In the case series, only 3 of the 6 patients could be followed up for more than 12 months. Nevertheless, we had achieved adequate occlusions in all those 3 cases. Thus, our case series may suggest the possible effectiveness of the treatment; however, long-term follow-up is essential to demonstrate the efficacy of this specific treatment for PcomA aneurysms with fetal-type PCAs. Second, introducing a Scepter catheter may stretch the PcomA and lead to damage in the perforating branches of the PcomA. Therefore, advancing a Scepter catheter into the PcomA requires great caution.
The double-balloon-assisted coiling technique can be an effective and feasible approach to protect the ICA and PcomA in the treatment of wide-necked PcomA aneurysms with fetal-type PCA.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.5469/neuroint.2022.00276.
Notes
Fund
None.
Ethics Statement
This retrospective study was conducted in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments and was approved by our institutional review board. The requirement for informed consent from patients was waived owing to the retrospective study design. We anonymized patient information as informed consent for publication was not obtained.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: YN. Analysis and interpretation: YN, TT, AH, and KS. Data collection: YN. Writing the article: YN. Critical revision of the article: TT, AH, and KS. Final approval of the article: YN. Statistical analysis: YN. Overall responsibility: YN.
