Endovascular Coiling of Fenestrated Vertebrobasilar Cerebral Aneurysms
Article information
Abstract
Fenestrated vertebrobasilar junction aneurysms are uncommon vascular lesions. Surgical intervention remains extremely challenging due to the deep location and complex anatomy with adjacent cranial nerves and perforator vessels. Endovascular approach is safer and generally accepted as the primary treatment method. Optimal angiographic projections with three-dimensional reconstructions to guide microcatheter selection remain vital to successfully treating aneurysms with challenging fenestration anatomy. This report details the endovascular methods in two cases of fenestrated vertebrobasilar junction aneurysms with different coiling techniques.
INTRODUCTION
Vertebrobasilar junction aneurysms are rare and complex lesions, and are often associated with basilar artery fenestration [1,2]. A surgical approach is typically deferred due to difficulty in achieving optimal exposure in addition to the complexity of brainstem anatomy and adjacent structures. Endovascular intervention is preferred for these aneurysms [3,4]. We report 2 cases of fenestrated vertebrobasilar junction aneurysms successfully treated with different endovascular coiling techniques.
CASE REPORT
Case 1
History and examination
A 59-year-old female was referred for management of an incidental cerebral aneurysm. Diagnostic angiography demonstrated fenestration of the proximal basilar artery with an associated broad-based 6×6×5 mm saccular aneurysm, arising from the proximal fenestration branch point and involving both fenestration branches. Ten days prior to intervention, the patient was started on dual antiplatelet therapy (aspirin 81 mg daily and clopidogrel 75 mg daily).
Treatment
Baseline standard, rotational three-dimensional (3D), and additional magnified oblique angiograms were obtained for optimal aneurysm visualization (Fig. 1A, B). To achieve proximal support, a 6 Fr Sofia Plus catheter (Terumo, Somerset, NJ, USA) was advanced to the right vertebral artery. Two paired 1.7 Fr Excelsior SL-10 (Stryker, Fremont, CA, USA) with 0.014-inch Synchro-10 Soft (Stryker) microsystems were introduced in tandem through the base catheter, and then each microsystem was navigated through separate basilar artery fenestration branches. A 3×21 mm endoluminal support stent was deployed across the aneurysm ostium through the dominant fenestration branch (Fig. 1C). The aneurysm was then selectively engaged through the stent interstices through the same dominant fenestration branch, and 4 Target 360 ULTRA and SOFT coils (Stryker) were deployed. The aneurysm was then selectively engaged through the second, non-dominant fenestration branch, where an additional 2 Target 360 NANO coils (Stryker) were detached into a different component of the aneurysm lumen and aneurysm embolization was complete with a final packing density of 18% (Fig. 1D).
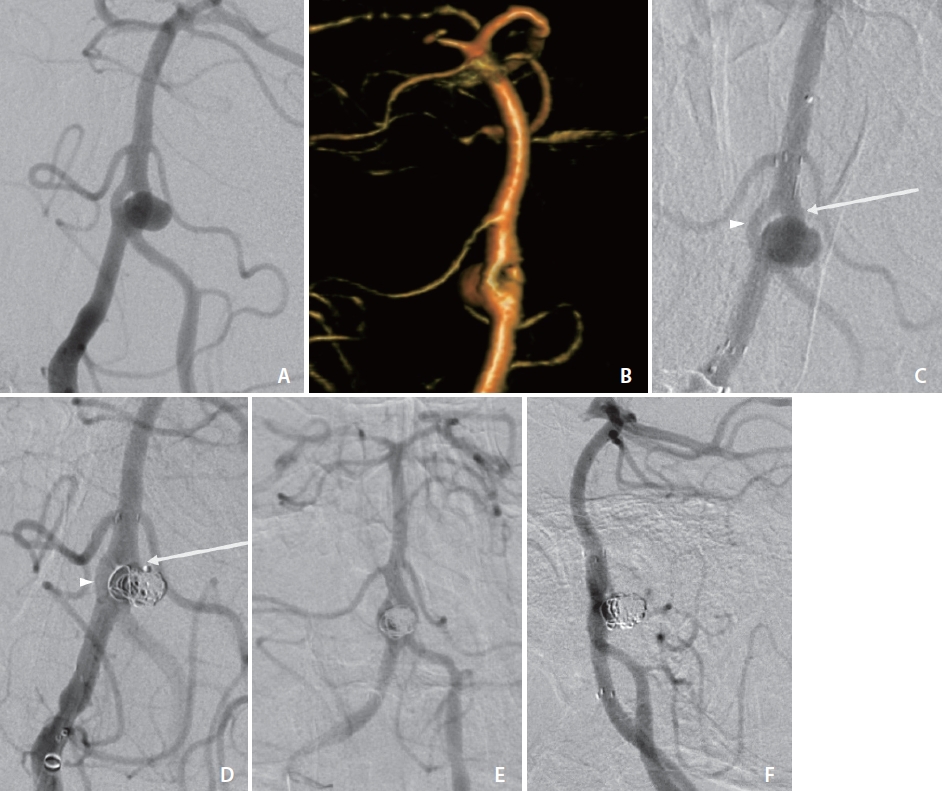
Angiogram (A) and three-dimensional volume rendering (B) with obliqued view of case 1 demonstrating broad-based aneurysm arising from the vertebrobasilar artery proximal fenestration branchpoint with involvement of both fenestration branches. (C) Angiogram of case 1 following deployment of Neuroform Atlas stent through the dominant fenestration limb (arrow) across the aneurysm ostium. Additional microcatheter remains within non-dominant fenestration limb (arrowhead). (D) Coil deployment in case 1 via the dominant fenestration limb (arrow) through the Atlas stent interstices and (not shown) subsequently through the non-dominant fenestration limb (arrowhead). Thirteen-month surveillance cerebral angiogram in frontal (E) and lateral (F) views demonstrating no contrast opacification of the aneurysm and stable stent positioning.
Outcome and follow-up
The patient tolerated the procedure well, without a change in neurological status, and was discharged the following day on dual antiplatelet therapy. A 13-month surveillance cerebral angiogram demonstrated no contrast opacification of the aneurysm (Fig. 1E, F).
Case 2
History and examination
A 53-year-old male with a history of methamphetamine and alcohol abuse was found down, altered, and confused by his wife. On initial presentation, the patient was hypertensive with systolic pressure in the 170–180 mmHg range with a Glasgow coma scale (GCS) of 11. Imaging demonstrated diffuse subarachnoid hemorrhage (Hunt & Hess grade 3 and modified Fisher grade 3) involving the pre-pontine and pre-medullary cisterns with a saccular vertebrobasilar junction aneurysm. An external ventricular drain was placed emergently for acute hydrocephalus. Cardiology was consulted for elevated troponins with ST elevation changes; however, a coronary angiogram with anticoagulation was deferred due to the unsecured cerebral aneurysm. In addition, the patient became acutely hypotensive, with systolic pressures in the 60s, and was started on pressors and broad-spectrum antibiotics for presumed septic shock. Following hemodynamic stabilization, the patient underwent diagnostic cerebral angiography, which revealed a narrow neck 5×4×8 mm saccular aneurysm involving the proximal basilar fenestration point.
Treatment
Baseline standard, rotational 3D, and additional magnified oblique angiograms were obtained for optimal aneurysm visualization (Fig. 2A, B). A 5 Fr Sofia was advanced to the distal vertebral artery for support. A 1.7 Fr Excelsior SL-10 with a 0.014-inch Synchro-2 microsystem was advanced to selectively engage the vertebrobasilar junction aneurysm ostia (Fig. 2C). A total of 8 Optima Complex 10 Soft and Super Soft (Balt, Irvine, CA, USA) and Target 360 Nano coils were deposited within the aneurysm for a packing density of 32%. Final angiograms demonstrated a marked decrease in aneurysm contrast opacification (Fig. 2D).
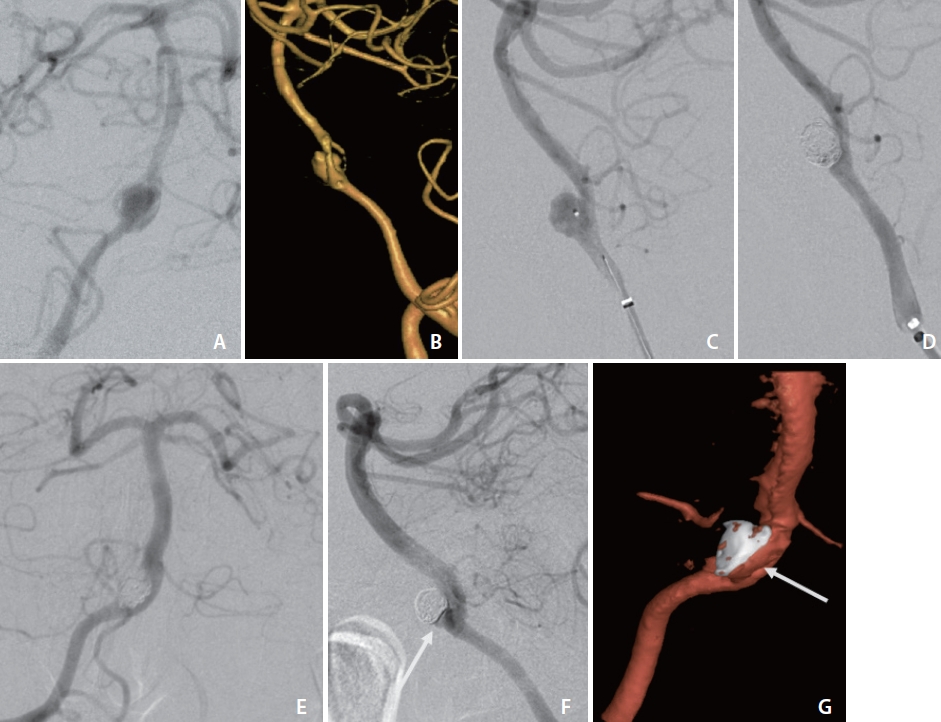
Angiogram (A) and three-dimensional (3D) volume rendering (B) of case 2 with obliqued view demonstrating narrow neck aneurysm arising from the vertebrobasilar artery proximal fenestration branchpoint, symmetric at bifurcation. Lateral view angiograms before (C) and following (D) primary coiling of case 2 with single microsystem. Eight-month surveillance cerebral angiograms in frontal (E) and lateral (F) views and 3D volume rendering (G) demonstrating contrast opacification of 1×2 mm neck remnant (arrows).
Outcome and follow-up
The patient tolerated the procedure well and returned to the neuro-intensive care unit for monitoring. The remainder of his complex care was predominantly related to substance use-induced cardiomyopathy and septic shock from aspiration pneumoniae. Repeat imaging for mental status change demonstrated new bilateral middle cerebral artery and anterior cerebral artery territory infarcts related to posthemorrhagic-related cerebral vasospasm. By post-procedure week 3, the patient was discharged to a rehabilitation facility with a mental status of GCS 14.
An 8-month surveillance cerebral angiogram demonstrated contrast opacification of 1×2 mm neck remnant (Fig. 2E–G). The patient had marked clinical improvement without focal deficits and the ability to function independently.
DISCUSSION
The prevalence of an observed fenestrated basilar artery varies depending on the diagnostic modality used: 2.3% in computed tomography angiography, 1.0% in magnetic resonance angiography, 0.6–1.7% in digital subtraction angiography, and 5.2% at autopsy [2,5,6]. Thin fenestration dividers, obscuration by an aneurysm, and competing flow from the contralateral vertebral artery make diagnosing fenestrations a challenge [3,7]. Histologic and morphologic studies of the fenestrated arteries demonstrate abnormal elastin composition within the proximal fenestration, locally absent tunica media, and proximal thinning and distal thickening of the subendothelium [7,8]. Furthermore, the medial blood vessel wall experiences higher pressure and shear stress at branching sites compared to the lateral walls [8,9]. This combination of structural vessel wall abnormality and altered hemodynamics predisposes aneurysm development at the fenestrated vertebrobasilar junction [2,4,6,10]. The reported incidence of vertebrobasilar junction aneurysms is rare at approximately 0.33% of all intracranial aneurysms [6]. In the presence of a vertebrobasilar junction aneurysm, a high index of suspicion must be maintained due to the association with abnormal fenestration in up to 70% of cases, which can alter the treatment approach [7,11].
As vertebrobasilar artery fenestration aneurysms are rare and complex lesions, an established classification has yet to be fully adopted. Different classification systems have been proposed based on fenestration morphology, location, and fenestration-aneurysm relationship; however, no dominant classification system has become clinically established. Most vertebrobasilar junction aneurysms are diagnosed following presentation of subarachnoid hemorrhage and are associated with a high risk of rebleeding and a high overall mortality rate with conservative treatment [10,12]. Operative treatment options for fenestrated vertebrobasilar aneurysms are complex and precarious. A surgical approach is limited due to difficulty in achieving optimal exposure at the proximal brainstem and the presence of various perforator vessels [7,10]. Despite being the preferred treatment modality, endovascular intervention remains challenging. Obtaining angiographic images for accurate diagnosis and preprocedural planning can be difficult due to overlapping structures on angiograms. 3D angiography is necessary for optimal planning and visualization of fenestration branches and of the aneurysm [3,4]. A few successful cases have been reported utilizing a variety of endovascular techniques, including stenting, coiling, balloon-assisted, liquid embolic, and flow diverting devices [1,3,4,13,14]. Regardless of technique, careful attention must be taken to preserve brain stem perforator arteries [5].
While primary coil embolization may be sufficiently obtain durable occlusion of saccular aneurysms with a narrow neck, proper occlusion of large, complex, wide-necked, or fusiform aneurysms are less likely [7,13,14]. However, stent-assisted coiling can effectively treat these morphologically-challenging aneurysms by using a stent as scaffolding [13,15]. In the presence of fenestrated cerebral arteries, adjunctive stenting will additionally reduce the likelihood of further aneurysm formation by mitigating the hemodynamic and structural wall changes of the fenestration limbs.
In conclusion, these uncommon lesions present unique diagnostic and therapeutic challenges due to the complex anatomy of fenestrated vertebrobasilar aneurysms. Obtaining optimal angiographic views remains vital for proper classification and preprocedural planning of these aneurysms. An endovascular treatment technique must be tailored to the characterization of these complex lesions in order to optimize success.
Notes
Fund
None.
Ethics Statement
Institutional Review Board approval for case reports is not required at our institution in California. Informed consent for publication was obtained from the patients.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Writing the article: ML, KH, and KC. Final approval of the article: RPPK.
