Fully Reversible Contrast-Induced Encephalopathy Mimicking Stroke after Flow Diverter Treatment of Carotid Cave Aneurysm
Article information
Abstract
Contrast-induced encephalopathy (CIE) is a rare complication of coronary and neurointerventional procedures. The condition is believed to arise from endothelial damage secondary to exposure to iodinated contrast media. A wide spectrum of clinical manifestations has been reported including seizures, cortical blindness, and focal neurological deficits. This report details the case of fully reversible CIE mimicking severe anterior circulation stroke in a 55-year-old female following elective endovascular treatment with a flow diverter of a carotid cave aneurysm. The patient was managed conservatively with intravenous hydration and steroids and showed an excellent prognosis with supportive management.
INTRODUCTION
Contrast-induced encephalopathy (CIE) is a rare condition complicating about 1–4% of neuroendovascular procedures [1,2]. It usually presents with rapidly deteriorating neurological deficits in the hours following angiographic procedures involving intra-arterial administration of iodinated contrast media [3]. The most commonly reported symptoms are headaches, seizures, and focal neurological deficits including transient global amnesia, cortical blindness, ophthalmoplegia, and hemiparesis [4]. Although mostly transient and reversible, the symptoms may be persistent with permanent sequelae and adverse clinical outcomes [5].
Herein, we report a case of CIE following flow diverter treatment that mimicked a severe anterior circulation stroke.
CASE REPORT
A 55-year-old Caucasian female without any clinical premorbidities was admitted to our hospital to undergo elective endovascular treatment of a carotid cave aneurysm of the left internal carotid artery (ICA), measuring approximately 9 mm in its biggest diameter (Fig. 1A, B).
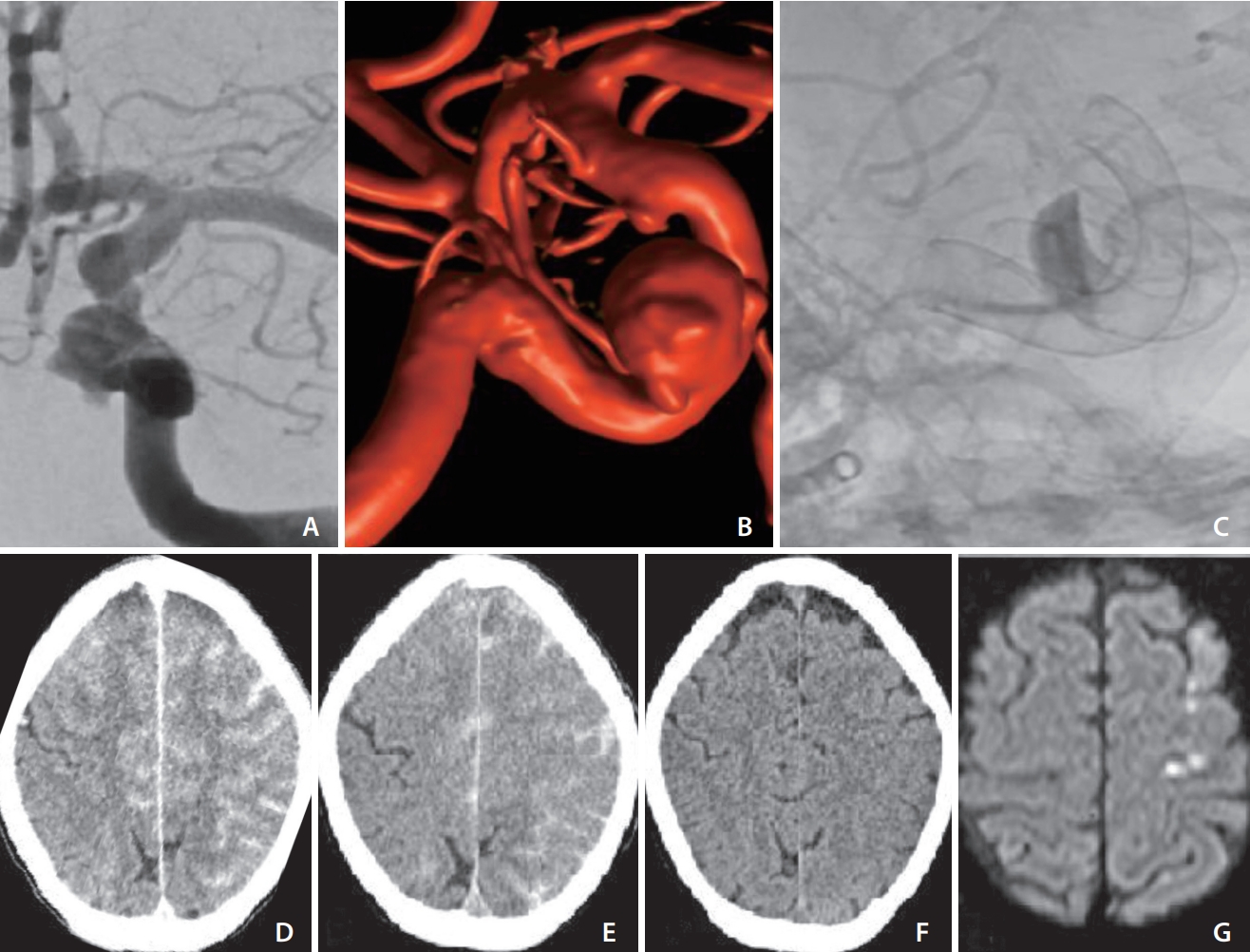
(A) Anteroposterior angiographic run showing carotid cave aneurysm of left internal carotid artery (ICA). (B) 3D rotational angiogram of the same aneurysm. (C) Unsubstracted image after flow diverter implantation showing intraaneurysmal contrast stagnation and also showing the superimposed flow diverter of the right ICA implanted 6 weeks earlier. (D) Post-procedural computed tomography (CT) showing sulcal hyperdensities mimicking subarachnoid hemorrhage. (E, F) Repeat non-contrast CT at 4 hours and 30 hours, respectively, showing gradual regression of previous findings and ruling out ischemic changes. (G) Magnetic resonance imaging on postprocedural day 5 showing a few punctate diffusion-weighted imaging hits.
The patient was under dual antiplatelet therapy with aspirin (100 mg/d) and clopidogrel (75 mg/d) following the treatment of a paraophthalmic aneurysm of the right ICA with a flow diverter (Derivo 2 Heal; Acandis, Pforzheim, Germany) 6 weeks earlier. A total of 70 mL of non-diluted contrast (Iohexol, Accupaque™ 300 mg/mL; GE Healthcare, Chicago, IL, USA) was used without any complications.
Induction in general anesthesia was performed, and standard monitoring was obtained. A triaxial system was introduced through a femoral access using a long sheath (Cerebase DA Guide Sheath; Cerenovus, New Brunswick, NJ, USA), an intermediate catheter (AXS Catalyst6; Stryker, Kalamazoo, MI, USA), and a 0.027 inch microcatheter (Neuroslider; Acandis). A 5x15 mm flow-diverter (Derivo 2; Acandis) was implanted with optimal wall apposition and a clear intraaneurysmal contrast stagnation (Fig. 1C).
Multiple angiographic runs and 3D rotational angiograms (3DRAs) were performed during the procedure, using a total of 125 mL non-diluted contrast (Iohexol, Accupaque™ 300 mg/mL; GE Healthcare).
Right after extubation, the patient presented with hemiparesis of the right upper and lower extremities with hemineglect towards the paretic side.
Approximately 45 minutes after the procedure, the patient became progressively aphasic, which rapidly developed into a complete receptive and expressive aphasia.
Non-contrast computed tomography (NCCT), computed tomography (CT)-angiography, and CT-perfusion were immediately performed to rule out stroke. The NCCT showed diffuse gyral hyperdensities mimicking sulcal subarachnoid hemorrhage in the entire left ICA territory with similar changes in the right anterior cerebral artery territory. Hyperdensity of the left basal ganglia was noticed (Fig. 1D). Subsequently, the patient was admitted to the neurosurgical intensive care unit where supportive therapy with intravenous (IV) fluids and corticosteroids (prednisolone 8 mg/d) was administered. No epileptic activity was observed. Four hours later, a repeat NCCT was obtained which showed a mild regression of the aforementioned changes (Fig. 1E).
Twenty hours after the procedure, the patient regained motor function in the lower extremity with persistent global aphasia and paresis of the right upper extremity.
A repeat NCCT performed 30 hours after the procedure showed complete disappearance of the earlier changes. No ischemic findings were noticed (Fig. 1F).
Over the course of the following 3 days, the symptoms gradually disappeared with the regaining of motor function of the right upper extremity.
The patient was discharged to the general ward on postprocedural day 4. On the fifth postprocedural day an magnetic resonance imaging (MRI) was performed, which showed a few punctate cortical diffusion-weighted imaging (DWI) lesions in the precentral, postcentral, and middle frontal Gyrus (Fig. 1G). No susceptibility foci were identified on T2*.
The patient was discharged from our hospital on the seventh postprocedural day. Her neurological examination at the time of discharge was unremarkable, except for dysgraphia. A neurological rehabilitation program was completed by the patient, which resulted in complete neurologic recovery at 3 weeks after discharge.
DISCUSSION
Pathophysiology and Risk Factors
CIE is a rare complication of diagnostic and interventional angiography procedures involving coronary and cerebral arteries. The pathophysiologic mechanism appears to be related to the disruption of the blood brain barrier after application of iodinated contrast media [6]. Non-ionic and low-osmolar contrast agents have also been reported to cause CIE [7]. These have been associated with a higher incidence of CIE of about 4% [4,8]. Iohexol, a low-osmolar non-ionic monomer, was used in this case.
It has been suggested that higher doses and lower temperatures of contrast media, kidney disorders, arterial hypertension, and acute cerebral infarction are risk factors for CIE [9]. Higher contrast dose and prior stroke were corroborated as risk factors in a recent meta-analysis [10]. However, lower doses of contrast agent have also been associated with CIE [11].
The doses reported in the literature varied widely depending on procedure type with volumes as low as 24 mL in a cerebral angiography case [6] and as high as 360 mL in a spinal angiography case [12]. Our patient received a total volume of contrast of 125 mL.
Clinical Manifestation
The hyperacute onset of symptoms after cerebral or coronary angiography and the reversibility of clinical findings are hallmarks in the diagnosis of CIE [13]. In a literature review on CIE following neurointerventional procedures, the most commonly reported symptom onset time was either immediately or within a few hours after angiography [14]. A prodromal onset pattern with gradual progression of symptoms over 17 hours has been reported in a case of a fatal irreversible CIE [15].
Late-onset imaging-confirmed encephalopathy cases with intervals of 1 week to 1 month following cerebral angiography have been attributed to posterior reversible encephalopathy syndrome and an inflammatory response to coating materials [16–18].
The clinical syndrome is usually reversible within 24–72 hours. Longer duration and irreversible or fatal outcomes, however, have also been reported [15,19].
A wide range of neurological deficits can occur depending on the arterial territory involved. Transient cortical blindness (TCB) is the most commonly reported manifestation of CIE related to coronary angiography. In cerebral angiography, TCB is mainly related to vertebral artery angiography [20].
Imaging Features
The main CT features of CIE are cortical swelling with poorly localized enhancement and hyperdensity of subarachnoid space [21] that may mimic subarachnoid hemorrhage. MRI usually shows T2/Flair hyperintensities in the involved territories with foci of diffusion-restriction in DWI [3]. Reversibility of these features in the subacute stage is the key to the diagnosis of CIE. This was also the case with our patient.
Literature Review
Different rates of CIE have been reported depending on procedure type, varying from 0.66% in coil embolization [22] to 1.7% in mechanical thrombectomy [1]. An incidence of 2.9% has been reported in a case series on TCB following coil embolization of posterior circulation aneurysms [23], suggesting a higher incidence of CIE in posterior circulation interventions. In comparison, CIE appears to be much less common in coronary angiography with reported incidences of 0.05–0.4% [24]. This could be explained by the frequent injections of large volumes of contrast directly into a single cerebral vessel in neurointervention [7].
Management and Prevention
Despite the lack of clinical evidence on the management of CIE, early supportive therapy with aggressive IV hydration and administration of steroids is the most commonly reported management strategy [25]. Our patient was managed conservatively with IV fluids and was monitored for epileptic activity in the early phase. No prophylactic anticonvulsant was administered.
Awareness of CIE and its typical clinical and radiological findings among interventionalists is essential for early recognition, correct diagnosis, and management. In patients with a history of CIE, avoidance of subsequent administrations of contrast media intra-arterially might be warranted. Stringent use of contrast in neurointerventional procedures avoiding multiple 3DRAs and distal access catheter injection seems advisable from our point of view.
Notes
Fund
None.
Ethics Statement
Institutional Review Board (IRB) approval for case reports is not required at our institution. We obtained individual written informed consents for the procedure and for publication.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: AVN and EAB. Analysis and interpretation: AVN and EAB. Data collection: AVN and EAB. Writing the article: AVN and EAB. Critical revision of the article: AVN and EAB. Final approval of the article: AVN and EAB.
