Carotid Artery Stenting for Asymptomatic Carotid Stenosis: What We Need to Know for Treatment Decision
Article information
Abstract
A clinical decision on the treatment of asymptomatic carotid stenosis is challenging, unlike symptomatic carotid stenosis. Carotid artery stenting (CAS) has been recommended as an alternative to carotid endarterectomy (CEA) based on the finding that the efficacy and safety of CAS were comparable to CEA in randomized trials. However, in some countries, CAS is often performed more frequently than CEA for asymptomatic carotid stenosis. Moreover, it has been recently reported that CAS is not superior to the best medical treatment in asymptomatic carotid stenosis. Due to these recent changes, the role of CAS in asymptomatic carotid stenosis should be revisited. When determining the treatment for asymptomatic carotid stenosis, one should consider several clinical factors including stenosis degree, patient life expectancy, stroke risk by medical treatment, availability of a vascular surgeon, high risk for CEA or CAS, and insurance coverage. This review aimed to present and pragmatically organize the information that is necessary for a clinical decision on CAS in asymptomatic carotid stenosis. In conclusion, although the traditional benefit of CAS is being revisited recently, it seems too early to conclude that CAS is no longer beneficial under intense and systemic medical treatment. Instead, a treatment strategy with CAS should evolve to select eligible or medically high-risk patients more precisely.
INTRODUCTION
Asymptomatic carotid stenosis >50% is responsible for about 10%–15% of all ischemic strokes [1]. Asymptomatic carotid stenosis can be managed medically or by revascularization treatment such as carotid endarterectomy (CEA) and carotid artery stenting (CAS). However, unlike symptomatic carotid stenosis, the optimal therapeutic approach to asymptomatic carotid stenosis is still controversial. First, studies on asymptomatic carotid stenosis and their results have been heterogeneous. Even though there are numerous guidelines on the management of asymptomatic carotid stenosis, the guidelines are also quite disparate [2]. For example, it is still questionable which cases of asymptomatic carotid stenosis require revascularization treatment and which revascularization method is optimal between CEA and CAS. Second, recent advancements in best medical treatment (BMT) have improved atherosclerosis-related outcomes in carotid stenosis [3]. In fact, the incidence of ipsilateral ischemic stroke with carotid stenosis was about 2% per year; however, it has decreased over 20 years to less than 1% per year [4,5]. Such an improvement might result in attenuating the relative efficacy of revascularization treatment in asymptomatic carotid stenosis [3,6,7]. Third, although CEA was principally recommended for revascularization of asymptomatic carotid stenosis, CAS has now been performed more frequently than CEA in some countries [8].
At this point, understanding the available evidence on revascularization treatment, especially focusing on CAS, can be helpful in setting up an optimal treatment strategy for asymptomatic carotid stenosis. Thus, this review aimed to pragmatically organize the information that is necessary for a clinical decision on CAS in asymptomatic carotid stenosis. Specifically, it tries to integrate every kind of result from related studies and published treatment guidelines. This review will primarily cover the following questions about asymptomatic carotid stenosis: (1) what is the current evidence for CAS; (2) what elements should be considered in deciding whether to perform revascularization treatment; (3) when is revascularization treatment necessary; and (4) when should we choose CAS preferentially over CEA.
EVIDENCE OF CAS FOR ASYMPTOMATIC CAROTID STENOSIS FROM LANDMARK TRIALS
CAS vs. CEA
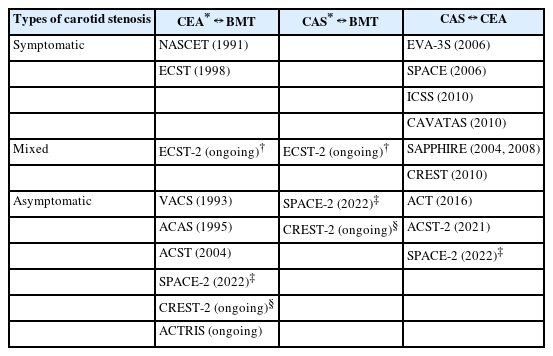
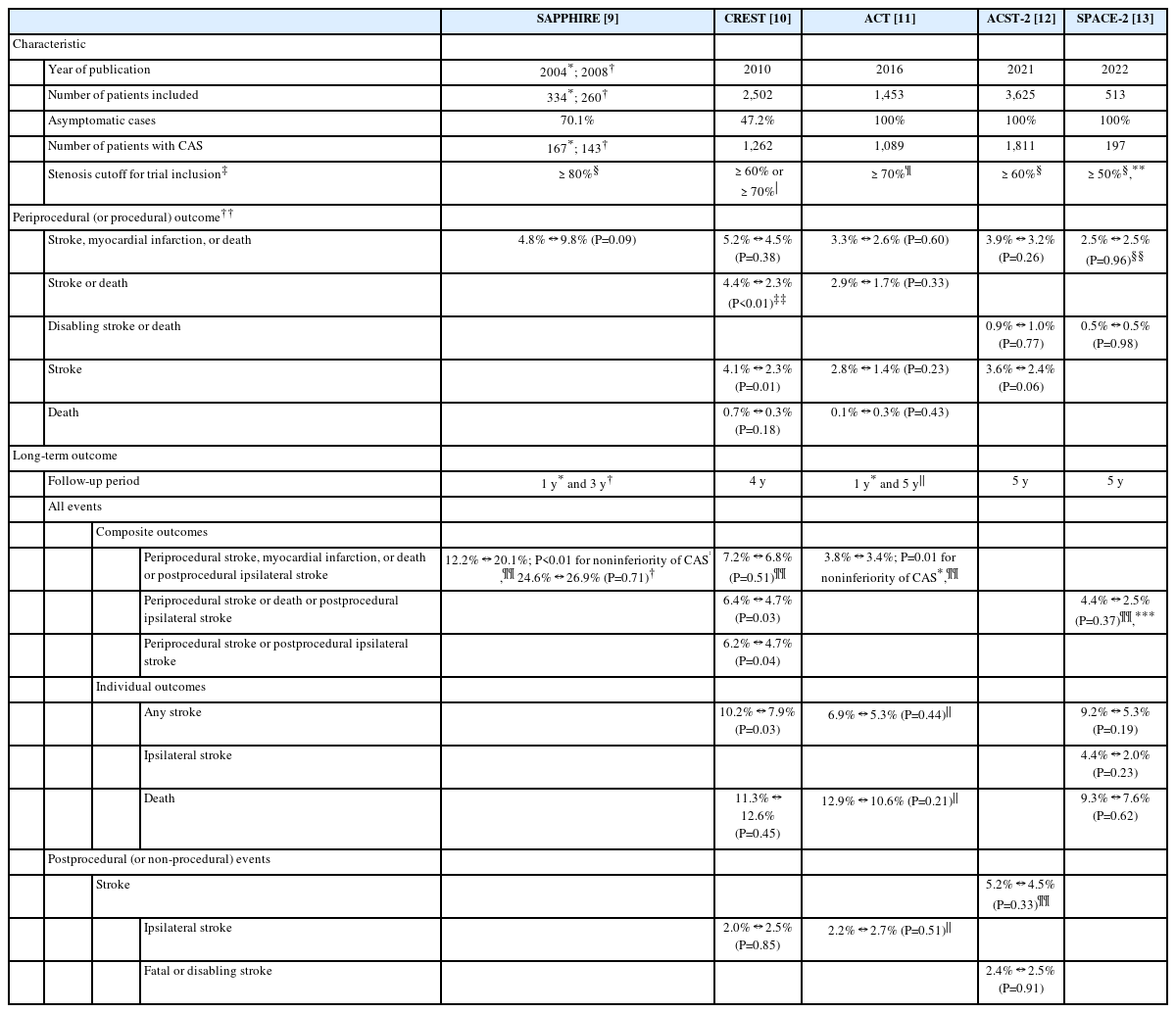
Five large randomized clinical trials directly compared CAS with CEA—Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE), Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST), Asymptomatic Carotid Trial (ACT), Asymptomatic Carotid Surgery Trial-2 (ACST-2), and Stent-Protected Angioplasty vs. Carotid Endarterectomy-2 (SPACE-2) (Table 1) [9-13]. The SAPPHIRE trial and CREST included patients with asymptomatic or symptomatic carotid stenosis, about 70% and 50% of whom, respectively, were asymptomatic (Table 2). The ACT, ACST-2, and SPACE-2 trial included exclusively patients with asymptomatic carotid stenosis. Stenosis cutoffs for trial inclusion were various from 50% to 80%.
Common periprocedural outcomes (stroke, myocardial infarction, or death within 30 days after the treatment procedure) were not significantly different between CAS and CEA in the SAPPHIRE trial (4.8% in CAS vs. 9.8% in CEA, P=0.09), CREST (5.2% vs. 4.5%; hazard ratio [HR], 1.18; 95% confidence interval [CI], 0.82–1.68; P=0.38), ACT (3.3% vs. 2.6%, P=0.60), and ACST-2 (3.9% vs. 3.2%, P=0.26) (Table 2). Event rates were around 5% after CAS in trials that partially included symptomatic carotid stenosis, which was slightly higher than other trials only with asymptomatic carotid stenosis (<4%). Most other periprocedural events (stroke or death, disabling stroke or death, stroke, and death) were not significantly different between CAS and CEA. However, periprocedural stroke or death and periprocedural stroke were significantly more frequent after CAS in the CREST.
For long-term outcomes, the trials principally evaluated the composite outcome of periprocedural stroke, myocardial infarction, or death or postprocedural ipsilateral stroke up to 4 years after the treatment procedure. In the SAPPHIRE trial and ACT, CAS was not inferior to CEA for the composite outcome during the first year (P=0.004 and P=0.01 for noninferiority under the 3%-point margin, respectively). Cumulative incidences of the composite outcome were not significantly different between CAS and CEA during 3–4 years of follow-up in the SAPPHIRE trial (24.6% vs. 26.9%, P=0.71, for 3 years) and CREST (7.2% vs. 6.8%, P=0.51, for 4 years). Other composite outcomes (periprocedural stroke or death or postprocedural ipsilateral stroke), individual outcomes (any stroke, ipsilateral stroke, and death), and postprocedural outcomes (stroke, ipsilateral stroke, and fatal or disabling stroke) were not significantly different between CAS and CEA in most of the trials. Only the CREST showed that stroke-related long-term outcomes were unfavorable after CAS. Any stroke (10.2% vs. 7.9%, P=0.03), periprocedural stroke or postprocedural ipsilateral stroke (6.2% vs. 4.7%, P=0.04), and periprocedural stroke or death or postprocedural ipsilateral stroke (6.4% vs. 4.7%, P=0.03) were significantly more frequent in CAS.
A recent meta-analysis including the SAPPHIRE trial, CREST, ACT, ACST-2, and SPACE-2 trial (only for 1-year outcome) also compared carotid revascularization treatments in asymptomatic carotid stenosis [14]. Compared with CEA, CAS showed no difference in periprocedural stroke, myocardial infarction, or death (odds ratio [OR], 1.13; 95% CI, 0.87–1.47; P=0.37) and periprocedural disabling stroke or death (OR, 0.91; 95% CI, 0.50–1.65; P=0.76). However, CAS had a higher risk of any stroke during the perioperative period (OR, 1.62; 95% CI, 1.16–2.24; P=0.004). Long-term stroke, myocardial infarction, or death were not significantly different between CAS and CEA (OR, 1.18; 95% CI, 0.95–1.48; P=0.14).
CAS vs. BMT
Up to now, only 1 randomized clinical trial directly compared CAS with BMT in asymptomatic carotid stenosis (Table 2). The SPACE-2 trial, originally, randomly allocated patients to the following 3 groups: CEA (exactly, CEA plus BMT), CAS (CAS plus BMT), and BMT (BMT alone) [15]. However, due to slow recruitment, the study protocol was amended to randomize patients to either CEA vs. BMT (SPACE-2a) or CAS vs. BMT (SPACE-2b) after the revascularization method was chosen by the treating physician. The SPACE-2 trial included patients whose extracranial carotid stenosis was ≥70% by European Carotid Surgery Trial (ECST) or ≥50% by North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria on ultrasonography. The carotid stenosis should be asymptomatic within the last 180 days. Notably, unlike other old trials, the SPACE-2 trial specified treatment targets for BMT, which included smoking cessation, limited alcohol consumption (e.g., <30 g/day for males), improved lipid profiles (e.g., low density lipoprotein <130 or 100 mg/dL according to comorbidities), body mass index <25 kg/m2 or 10% weight loss, blood pressure ≤130/85 or 130/80 mmHg, HbA1c <7%, and physical activity ≥30 minutes at least 3 times a week. A planned number of patients (3,640) could not be recruited due to slow recruitment [13]. Finally, a total of 513 patients were included, then 197 and 113 were enrolled in CAS and BMT, respectively. Primary outcomes (periprocedural any stroke or death or postprocedural ipsilateral ischemic stroke within 5 years) were not significantly different between CAS and BMT (4.4% in CAS vs. 3.1% in BMT; HR, 1.55; 95% CI, 0.41–5.85; P=0.52). During the 5-year follow-up period, ipsilateral ischemic stroke (4.4% vs. 3.1%; HR, 1.55; 95% CI, 0.41–5.85; P=0.52), any stroke (9.8% vs. 6.5%; HR, 1.66; 95% CI, 0.65–4.20; P=0.29), and death (9.3% vs. 8.0%; HR, 1.05; 95% CI, 0.45–2.48; P=0.91) were not significantly different, either.
Interpretation
First, for revascularization procedures on asymptomatic carotid stenosis, CAS seems to be comparable to CEA for 4 reasons: (1) Noninferiority of CAS to CEA was demonstrated in the SAPPHIRE trial and ACT, although it was just for the 1-year outcome; (2) A recent meta-analysis also showed that CAS and CEA are comparable in periprocedural or long-term outcomes [14]; (3) Some periprocedural and long-term outcomes were unfavorable to CAS in the CREST. However, it should be cautiously interpreted because the CREST included symptomatic carotid stenosis even in >50% of the study population. The nature of the CREST might be somewhat different from other trials that included mostly or exclusively asymptomatic carotid stenosis. Thus, the unfavorable outcomes found in the CREST might not be applicable to asymptomatic carotid stenosis as they were; (4) Comprehensive interpretation can be challenging because every trial had different types of primary or main outcomes. However, cumulative incidences of the various periprocedural and long-term outcomes were consistently comparable between CAS and CEA in all trials, except for the CREST.
Second, CAS might not be superior to the intensive medical treatment of asymptomatic carotid stenosis, specifically for ≥50% of stenosis. It is completely from the finding of the SPACE-2 trial, which showed that all kinds of common periprocedural and long-term outcomes were not different between CAS and BMT. In the SPACE-2 trial, remarkably, CEA was not superior to BMT either. The SPACE-2 trial was the first to set the specifically-designed BMT, which can be a key factor in the SPACE-2 trial. In fact, the annual risk of stroke-related events has steadily decreased over time. In the early period of clinical trials in the 1980s–1990s, the annual risk of stroke was minimally 4% [3,16]. However, in the 2000s, the annual risk of stroke was merely <2% irrespective of stenosis degree. In the SPACE-2 trial, the annual risk of stroke was extremely low, merely 0.6%/year [13]. Probably due to the advancement of medical strategies in the primary prevention of stroke, medical treatment seems to have been much more effective also in asymptomatic carotid stenosis. In fact, the quality and intensity of medical treatment are now widely emphasized in asymptomatic carotid stenosis, like in the case of intracranial stenosis [3]. Medical treatment was specified and intense also in the ongoing CREST-2 trial, where all patients receive detailed lifestyle coaching including smoking cessation, lowering body mass index or weight loss, and regular physical activities in addition to strong medication [17]. However, interpretation of the SPACE-2 trial should be cautious for the following reasons: (1) Achieving a sufficient level of medical treatment is not easy in real clinical practice. Medical treatment under the randomized trial could lead to better BMT adherence than in real clinical practice. Even in the SPACE-2 trial, blood pressure adjustment and weight reduction were achieved only in 25% of the study population; (2) In the SPACE-2 trial, not just a few patients (about 36%) had non-high-grade carotid stenosis (70%–79% by ECST criteria, which corresponds to 50%–69% in NASCET criteria) [13]. Such a mild nature of carotid stenosis might exaggerate the effect of BMT; (3) It should be kept in mind that the SPACE-2 trial was early terminated with a far fewer number of patients than planned.
PRACTICAL DECISION-MAKING FACTORS FOR CAS IN ASYMPTOMATIC CAROTID STENOSIS
To decide whether to perform CAS, a few clinical factors should be considered: (1) stenosis degree; (2) patient’s life expectancy; (3) stroke risk by medical treatment; (4) availability of a vascular surgeon; (5) high risk for CEA or CAS; and (6) insurance coverage (optional, only for a particular country) (Table 3). The crucial factors are the first 3 elements, which are all for the direct assessment of the risks and benefits of revascularization treatment. In addition, the availability of a vascular surgeon and the high risk for CEA or CAS are also important in determining the type of revascularization treatment.

Practical decision-making factors and related recommendations for revascularization treatment of asymptomatic carotid stenosis
Stenosis Degree
Stenosis degree is the most fundamental criterion to determine the necessity of revascularization treatment in asymptomatic carotid stenosis. Stenosis degree for revascularization treatment is highly dependent on the inclusion cutoff implemented in landmark trials. Randomized trials for asymptomatic carotid stenosis set the cutoff as ≥50%–80% (Table 2) [10-13]. Considering the SPACE-2 trial did not show a clear benefit of CAS in patients with asymptomatic carotid stenosis ≥50%, the relevant stenosis degree can be narrowed to ≥60%–80% from ≥50%–80%. All guidelines, in fact, have recommended CAS for asymptomatic carotid stenosis ≥60%–80% (Table 4) [1,18-29]. Guidelines from American Heart Association/ American Stroke Association (AHA/ASA, 2011; 2014), European Society for Vascular Surgeon (ESVS, 2017), German/ Austrian society (2020), and Society of Vascular Surgery (SVS, 2021) clearly indicated stenosis degree eligible to CAS. On the contrary, the ESVS (2009) guideline, the European Stroke of Cardiology (ESC, 2011) guideline, and the European Stroke Organization (ESO, 2011) guideline recommended CAS just as an alternative to CEA without specifying stenosis degree for CAS. In those guidelines, the stenosis degree for CAS was merely estimated from that for CEA.
In the AHA/ASA guidelines, stenosis degree for CAS was basically indicated as ≥60%, which was determined by catheter angiography. With other diagnostic modalities, CAS was also recommended for asymptomatic carotid stenosis ≥70% by duplex ultrasonography (DUS) or ≥80% by computed tomography angiography (CTA) or magnetic resonance angiography (MRA) if the stenosis is ≥50%–69% on DUS (Table 5). Like this, especially in the AHA/ASA (2011; 2014) guidelines, an eligible stenosis degree can be dependent on the type of diagnostic modality. DUS is the most widely-used and preferable modality to assess stenosis degree [28]. However, catheter angiography was also utilized in the CREST and ACT and is recommended in the AHA/ASA (2011; 2014) guidelines. CTA or MRA can be used equally with DUS based on the ESC (2011) guideline and the ESVS (2017) guideline. CTA or MRA can be also added for equivocal or inaccurate stenosis on DUS according to the AHA/ASA (2011; 2014) guidelines, the German/Austrian (2020) guideline, and the SVS (2021) guideline. Roughly, for asymptomatic carotid stenosis ≥60%, all kinds of modalities are possible to evaluate stenosis degree. For ≥70%, DUS is mostly preferable to evaluate stenosis degree. However, in real practice, evaluating modalities is not strictly limited in the decision process of CAS. Thus, DUS and non-invasive vascular images such as CTA or MRA are commonly performed together.
Although stenosis degree for CAS has not been consistent throughout historical trials and guidelines, the most recent 4 guidelines primarily recommend CEA for asymptomatic carotid stenosis ≥60% or ≥70% (Table 3) [1,26,28,29]. European guidelines preferably adopted ≥60% as cutoff, whereas the cutoff was ≥ (or >) 70% in American guidelines. However, the rationale for the difference has not exactly been determined. Stenosis degrees for CAS were not different from those for CEA. Exceptionally, asymptomatic carotid stenosis ≥60% by catheter angiography can be eligible for CAS in the AHA/ASA (2014) guideline.
Patient’s Life Expectancy
Patient’s life expectancy has been a main consideration in the decision of revascularization treatment in asymptomatic carotid stenosis, although it is easily overlooked. Most of the randomized trials had a specific criterion of patient’s life expectancy for inclusion—minimally 1 year in the SAPPHIRE trial, 3 years in the ACT, and 5 years in the CREST and SPACE-2 trial [9-11,13]. Recent guidelines also commonly recommend performing revascularization treatment on a patient with a life expectancy ≥3 or 5 years (Table 3).
Stroke Risk by Medical Treatment
Preventing the development of future stroke is a primary goal of revascularization treatment of carotid stenosis. The risk of occurrence of future stroke by medical treatment should be more thoroughly assessed in asymptomatic carotid stenosis than in symptomatic carotid stenosis. Although the stenosis degree basically suggests the risk of future stroke [30], there are a variety of clinical and imaging features associated with the risk of future stroke in asymptomatic carotid stenosis (Table 6). They include: (1) stenosis progression; (2) plaque characteristics including morphology, size, and vulnerability; (3) accompanied stroke-related conditions; and (4) microembolization. The risk of future stroke can be doubled if stenosis progresses by 10% [31-33]. Various imaging features such as intraplaque hemorrhage, plaque ulceration, plaque neovascularity, thin/ruptured fibrous cap, and presence of a lipid-rich necrotic core are closely associated with a vulnerable plaque [34]. In the case of echolucent plaque or intraplaque hemorrhage, the risk increases by more than 2 or 3 times [35-37]. For patients with silent infarction or contralateral transient cerebral ischemic attack or stroke, the risk can be 3 times higher [38,39]. Impaired cerebrovascular reserve or spontaneous microembolization could increase the risk even by 6 times or more [40-42]. In the most recent guidelines, European guidelines especially recommend considering the risk of future stroke in the decision-making process of revascularization treatment (Table 3) [1,29]. If a patient with asymptomatic carotid stenosis has at least 1 of those features that can increase the risk of future stroke, revascularization treatment should be actively considered [1,2].
High Risk for CEA or CAS
Since CAS is an alternative to patients ineligible for CEA, high-risk features for CEA should be checked (Table 7). High cervical lesions and complicated surgical fields are non-vascular features for high-risk CEA. Commonly, carotid lesions above C2 level are regarded to be inappropriate to CEA. High carotid exposure can increase the risk of cranial nerve injury particularly, such as the vagus nerve. Previous neck surgery or irradiation and presence of tracheal stroma can accompany fibrotic or scarred changes of surrounding connective tissues, which makes surgical dissection more tough during CEA. Spinal immobility by previous cervical fusion, arthritis, or kyphosis and even a short neck with obesity can also make CEA more challenging. As CEA has an apparent risk of cranial nerve injury, patients with a contralateral vocal cord injury or laryngeal nerve palsy are regarded to be ineligible for CEA.
The influence of contralateral carotid occlusion on CEA is a bit conflicting. Several studies and a meta-analysis showed that contralateral carotid occlusion significantly increased the perioperative stroke risk after CEA [43]. However, the increase was very modest. Moreover, in another study, strokefree survival after CEA was comparable between patients with contralateral carotid occlusion and those without [44]. The presence of a tandem lesion can be associated with a higher risk of stroke after CEA, which might be due to the increased chance of hemodynamic compromise during CEA and general anesthesia. Tortuosity of the aortic arch, common carotid artery, and internal carotid artery is not only associated with technical failure, but also increases the risk of stroke and death after CAS [45,46]. Specifically, in the tortuous internal carotid artery, it can be difficult to position a distal embolic protection device to secure the proper location to place a carotid stent. In addition, the action of a distal embolic protection device might be less effective in the tortuous internal carotid artery. Atherosclerotic burden can be directly associated with the periprocedural risk in CAS. Catheter manipulation in the aortic arch and target arteries with high atherosclerotic burden is associated with embolic stroke [46]. Lesion morphology also significantly affects periprocedural outcomes in CAS. Various conditions such as tight stenosis, heavily-calcified carotid stenosis, and a long complex lesion could disturb the passage of endovascular devices across the lesion [45,46]. Heavily-calcified plaque is also associated with stent fracture or deformation [47]. Long and complex stenosis can increase the risk of embolic stroke during endovascular manipulation [45]. On the contrary, lesion morphology is not generally associated with treatment outcomes after CEA.
Patients with an older age, usually >80 years old, are associated with a higher risk of stroke in CAS than in CEA [10,48]. In the CREST, the risk of stroke was higher in patients aged >70 years after CAS [10]. Older age is also associated with a higher risk of stroke in CEA. In the ACST-1, the benefits of CEA were only observed in patients <75 years, and not in patients ≥75 years (P for interaction by age=0.04) [49]. However, this might be due to older patients having more comorbidities that affect the risk of stroke after CEA [28]. Associated comorbidities including severe cardiac and pulmonary diseases and renal failure are expected to increase the risk of stroke after CEA and CAS. However, there is little evidence to support the association. As CAS is associated with a relatively lower risk of cardiac or pulmonary events than CEA, it is preferred over CEA when severe cardiac or pulmonary comorbidities are present. Chronic renal insufficiency could also affect the risk of stroke after CEA and CAS [50]. Because the risk of stroke is also increased even after CEA, medical treatment can be preferred to CEA or CAS in chronic renal insufficiency.
The position of CAS is somewhat limited in asymptomatic carotid stenosis. In all the most recent guidelines, CEA is a revascularization method preferred or a treatment of choice for asymptomatic carotid stenosis (Table 3). CAS is only allowed in cases where CEA is high-risk or not suitable. In contrast to the clear relationship between CEA and CAS, the role of CAS has not yet been settled in comparison with medical treatment alone. The AHA/ASA (2014) guideline only commented on the lack of evidence about the effectiveness of CAS compared with medical treatment alone [26]. Based on the short-term interim results of the SPACE-2 trial, the ESO (2021) guideline recommended against CAS as a routine alternative to medical treatment alone [29]. However, despite the recent results of the SPACE-2 trial, it seems not appropriate yet to reduce the role of CAS in asymptomatic carotid stenosis. Considering the limitations of the SPACE-2 trial, further studies should be necessary to confirm it.
Overall, periprocedural risk should be low around <3% for CAS, which is equal to perioperative risk for CEA. The ESO (2021) guideline recommends that periprocedural risk should be <2% because complication rates have improved in recent years.
SUMMARY AND CONCLUSIONS
Regarding outcomes including efficacy and safety, CAS was comparable to CEA in asymptomatic carotid stenosis. However, with older studies, it appears unwarranted to conclude that CAS is better than BMT. Furthermore, with the recent direct finding that CAS is not superior to BMT from the SPACE-2 trial, it looks like the role of CAS in asymptomatic carotid stenosis should be re-evaluated. However, considering some limitations of the SPACE-2 trial, it seems too early to assert that CAS is no longer beneficial under the intense and systematic medical treatment in asymptomatic carotid stenosis. Instead, we should revisit the clinical and imaging features associated with high risk of future stroke in asymptomatic carotid stenosis. In addition, further studies are necessary to optimize the eligibility to CAS by solving unmet problems related to the high-risk features. Now in practice, one should assess the high-risk features more thoroughly in deciding to perform CAS in asymptomatic carotid stenosis. Guidelines will be certainly revised by further studies, which are expected to indicate more precise eligibility for CAS instead of the futility in asymptomatic carotid stenosis.
Notes
Fund
None.
Ethics Statement
This study was exempted from the review by the institutional ethics committee. This article does not include any information that may identify the person.
Conflicts of Interest
JB has been the assistant editor of Neurointervention since 2018. No potential conflict of interest relevant to this article was reported.