A Review of Endovascular Treatment for Posterior Circulation Strokes
Article information
Abstract
Mechanical thrombectomy for acute posterior circulation strokes (PCSs) is recommended based on evidence from anterior circulation strokes (ACSs). Two recent randomized controlled trials showed that endovascular treatment (EVT) leads to better functional outcomes than those of the best medical care. However, many studies have shown that patients undergoing PC-EVT have a higher rate of futile recanalization than those undergoing AC-EVT. The characteristics and outcomes of PC-EVT may differ according to the pathological mechanisms, including cardioembolism, intracranial atherosclerosis, and tandem vertebrobasilar occlusion. We reviewed PC-EVT outcomes reported in recent studies and discussed technical considerations for maximizing treatment efficacy according to the etiology of a PCS.
INTRODUCTION
Posterior circulation strokes (PCSs) account for approximately 20% of all strokes; however, PC-large vessel occlusions (LVOs) are rare, representing only 1% of all ischemic strokes and 5% of all LVOs [1-3]. PCSs, particularly those due to acute basilar artery occlusions (BAOs), are devastating with high mortality. Good clinical outcomes occur in approximately 20% of patients despite the advanced care, with a lower rate than that for anterior circulation strokes (ACSs) after revascularization [4-6]. Unlike hemispheric ischemia, the PCS clinical presentation can be varying and ambiguous, resulting in delays in clinical neurological evaluation and identification [7]. Furthermore, because the BA serves as the primary source of arterial supply to the brainstem, clinical consequences can be devastating, with high rates of poor outcomes in patients with a BAO [8].
Endovascular treatment (EVT) based on mechanical thrombectomy (MT) has become the gold standard treatment for LVOs in the AC. In PC-LVOs, EVT has been associated with improved functional outcomes in a large prospective multicenter registry [9], and successful reperfusion has been associated with favorable outcomes in multiple studies and a recent systematic review [10-13]. However, 2 recent randomized controlled trials (RCTs), hindered by excessive crossover between treatment arms and poor recruitment, failed to show the therapeutic efficacy of EVT [14,15]. Eventually, 2 additional RCTs have demonstrated positive results for PC-EVT [16,17]. However, despite the promising results, more than half of the enrolled patients did not achieve favorable outcomes in the 2 recent RCTs [18]. Moreover, many recent studies have shown that patients undergoing PC-EVT have a higher rate of futile recanalization than that of those undergoing AC-EVT [19-21].
The characteristics and outcomes of PCS-EVT depend on the underlying pathological mechanism. Patients with PCS undergoing EVT have different etiologies such as cardioembolism, intracranial atherosclerosis (ICAS), and artery-to-artery embolism (tandem occlusion) [8,22-24]. These etiological factors should be considered for planning the PC-EVT. We believe that EVT should be tailored according to the presumed etiology when possible. However, the best MT strategy, including the first-line technique for the PCS treatment, remains unclear and may vary according to ICAS vs. embolic etiology. In this study, we first reviewed the recent RCTs on PC-EVT and then discussed its outcomes and technical considerations based on the etiology of PCS.
FOUR RCTS FOR EVT IN PC-LVOS
EVT has become the standard treatment for an acute ischemic stroke due to an AC-LVO [25]. Although it is routinely performed in PC-LVOs, owing to its success in treating AC-LVOs, a high-level evidence to support its superiority over standard medical therapy is lacking [26]. Over the last 3 years, 4 RCTs have been published regarding the efficacy and safety of EVT compared with those of medical therapy for acute ischemic stroke due to vertebrobasilar artery occlusion (Tables 1, 2) [14-17]. Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment (BEST) was a multicenter, randomized, and open-label trial with a blinded outcome assessment of thrombectomy in patients presenting within 8 hours of vertebrobasilar occlusion at 28 centers in China. The primary outcome was a modified Rankin scale (mRS) score of 3 or lower at 90 days [14]. The primary and secondary safety outcomes were mortality at 90 days and rates of symptomatic intracranial hemorrhage (sICH). This trial was terminated soon after 131 patients were randomly assigned (66 in the intervention group and 65 in the control group) because of the high crossover rate and poor recruitment. For the primary endpoint analysis, no difference in favorable outcomes in patients receiving EVT (42%) compared with the outcomes in those receiving standard medical therapy alone (32%) was detected (adjusted odds ratio [OR], 1.74; 95% confidence interval [CI], 0.81–3.74; P=0.23). The 90-day mortality rate was similar among the groups (33% in the intervention group vs. 38% in the control group; P=0.54) despite a numerically higher prevalence of sICH in the intervention group [14].
The Basilar Artery International Cooperation Study (BASICS) trial was conducted at 23 centers in 7 countries, and the enrolled patients were presented within 6 hours from a stroke due to a BAO [15]. The original inclusion criteria were patients aged <85 years old and National Institutes of Health Stroke Scale (NIHSS) scores of ≥10. However, because of the slow enrollment and the uncertainty suggested by the screening data, the inclusion criteria were expanded to include patients aged >85 years old and those with NIHSS scores of <10. The primary outcome was an mRS score of 0–3 at 90 days. Primary safety outcomes were sICH and mortality at 90 days. In total, 300 patients were enrolled in the study (154 in the EVT group and 146 in the medical care group). The primary outcome was achieved in 44.2% and 37.7% of the EVT and medical care groups, respectively, with no significant difference (risk ratio [RR], 1.18; 95% CI, 0.92–1.50). The sICH and mortality rates did not differ between the 2 groups [15]. Both the BEST and BASICS trials were neutral in demonstrating the superiority of EVT for BAOs despite a direction of the treatment effectivity. The BEST and BASICS trials were pivotal in defining patient selection for EVT, refining the primary outcomes and sample sizes of the 2 subsequent RCTs [27].
Two recent added RCTs have shown positive results. Basilar Artery Occlusion Chinese Endovascular (BAOCHE) was a randomized trial comparing EVT with the best medical management (BMM) in 217 patients with a BAO presenting 6–24 hours from the symptoms onset in Chinese stroke centers between 2016 and 2022 [16]. In contrast to BEST and BASICS, the BAOCHE trial utilized clinical severity criteria of an NIHSS score of >6, imaging-based inclusion criteria of PC-Acute Stroke Prognosis Early Computed Tomography Score (ASPECTS) of ≥6, and a pons midbrain index score of ≤2. The trial was halted after an interim analysis demonstrated a significantly higher proportion of patients achieving the primary outcome (mRS score: 0–3) in the EVT group compared with that in the control group (46% vs. 24%; adjusted RR, 1.81; 95% CI, 1.26–2.6; P<0.001) [16].
Endovascular Treatment for Acute Basilar Artery Occlusion (ATTENTION) was a randomized trial comparing EVT with medical therapy in 340 patients with a BAO presenting within 12 hours from the onset in 36 Chinese stroke centers between 2021 and 2022 [17]. The ATTENTION trial had both a clinical severity criterion of an NIHSS score of >10 and an imaging-based inclusion criterion of PC-ASPECTS of ≥6. The primary end point of good functional outcome was defined as an mRS score of 0–3, which was 2-fold greater in the EVT group compared with that in the BMM group (46% vs. 23%; adjusted OR, 2.06; 95% CI, 1.46–2.91; P<0.001) [17].
The success of BAOCHE and ATTENTION, in contrast to BEST and BASICS, which failed to reach the significance of better functional outcomes in EVT, might be explained by the differences in patient selection and populations. Both the BAOCHE and ATTENTION trials applied specific inclusion criteria composed of baseline clinical severity and infarct volumes estimated using the PC-ASPECTS and pons-midbrain index. The 2 trials included patients with a baseline NIHSS score of >6 (in BAOCHE) or 10 (in ATTENTION) and excluded patients with a PC-ASPECTS score of <6 (or <8 in patients aged ≥80 years old in ATTENTION) and a pons-midbrain index score of >2 (in BAOCHE), whereas the 2 prior RCTs lacked a quantitative assessment of ischemic injury on non-contrast CT [27].
Regarding the failures of BEST and BASICS, some factors should be considered. First, triage of PCS from emergent LVO might not have been tailored to maximize EVT outcomes [28]. For maximal efficacy of reperfusion therapy, the extent of the infarct core should be limited, while there should be a large penumbral area relevant to clinical severity [29,30]. Although the PC may be more prone to a Willisian collateral failure, resulting in a fast infarct growth [31], even a small infarction might be critical because of the higher density of neural pathways and nuclei located in the PC [32,33]. Accordingly, the probability of futile recanalization might have increased in the 2 prior RCTs. Furthermore, there were no clinical severity criteria for BEST, and NIHSS scores of ≥10 in the BASICS criteria were later discarded. Second, the high crossover rate and the decrease in the average rate of valid recruitment per center occurred because of family member resistance to conservative treatment in the BEST trial and perhaps in BASICS [28]. Therefore, many of patients were treated outside the trial (29% of screened patients in BASICS and 55% in BEST), potentially causing bias [34].
Both the BAOCHE and ATTENTION trials had differences from the BASICS and BEST trials. First, they were primarily conducted in China, which might limit the generalizability of their results to western populations [18]. ICAS was the cause of stroke in nearly half of the patients enrolled in BAOCHE (66%) and ATTENTION (44%), higher than its rates in BEST (52%) and BASICS (35%) [27]. Additionally, intracranial angioplasty and stenting were used more frequently in the EVT arm of the ATTENTION compared with in the BASICS trial (40% [88/221] vs. 17% [26/154], respectively). In BAOCHE, 55% of the EVT patients were treated with angioplasty and stenting. Second, intra-venous thrombolysis (IVT) rates in ATTENTION and BAOCHE were lower (medical therapy groups: 34% and 21%, respectively), related to the later-time window [16,17,27]. In contrast, in BASICS, nearly 80% of patients were treated with IVT in both treatment groups, and patients in the BMM group showed an unexpectedly high good outcome rate (38% with an mRS score of 0–3). Third, across the 4 BAO RCTs, over two-thirds of the recruited patients were males. This might reflect sex differences in harboring LVOs and its related vascular risk factors. Whether this difference reflected the challenges of recruiting women in RCTs or discerning their eligibility for EVT was unclear [27].
A recent meta-analysis of 4 PCS RCTs including the BEST, BASICS, BAOCHE, and ATTENTION trials has showed that EVT has a significantly higher rate of good functional outcome and independence at 90 days compared with that of medical therapy alone [18]. However, despite these high rates of favorable outcomes, 55% of patients in the EVT arm had severe disability (mRS score: 4–5) or had died by the 90-day follow-up visit [18]. Meanwhile, reperfusion success to thrombolysis in cerebral infarction (TICI) score of 2b/3 was achieved in 85% of the EVT patients in this meta-analysis. This was higher than the 71% TICI score of 2b/3 reperfusion success reported for AC-LVOs [18]. The futile recanalization of PC-LVOs may not only require more refined imaging that identifies areas of irreversible brain injury but also require tailored rapid-successful reperfusion strategy. Next, we reviewed the characteristics of PCS-EVT and discussed technical considerations for improving the treatment efficacy of PC-EVT according to the etiology.
PCS-EVT VS. ACS-EVT: TECHNICAL PERSPECTIVE
There are several considerations for EVT in treating PC-LVOs. The PC differs from AC in terms of anatomy, including the caliber and tortuosity of the vessels. This can pose technical challenges in MT.
The diameter of the vertebral arteries (VAs) is usually <3 mm with a relatively smaller caliber compared with that of the internal carotid arteries (4–5 mm) and common carotid arteries (6–7 mm) [35,36]. For EVT, large-bore aspiration systems afford benefits compared with those of smaller systems; however, the reduced diameter of the VA may pose a limit [33]. Many neurointerventionists prefer an 8-Fr guide catheter in AC-LVO; however, smaller guides of 6 Fr in the distal VA still prove adequate support in advancing a 5-Fr aspiration catheter into the BA.
The VA commonly originates from the subclavian arteries with an acute take-off angle, while the internal carotid arteries extend directly from the parent common carotid arteries. Tortuous V1 (pre-foraminal) segment anatomy impairs the best positioning of the guiding catheter and subsequent distal progression of catheters. Therefore, it is important to recognize vascular tortuosity of first segment of the VA like coiling and kinking that may be a barrier to intracranial access on pre-treatment imaging. In the case of VA orifice with acute take-off angle, an angled diagnostic catheter may be helpful in navigating the microwire and microcatheter system. In the case of tortuous VA, the use of intermediate catheter may help to overcome cervical VA tortuosity, providing adequate stability for microcatheter vessel selection into intracranial vasculature. Furthermore, use of a 0.014 or 0.018-inch buddy-wire or a large-caliber coronary guide catheter placed in the subclavian artery may help to increase proximal support [37].
The anatomical differences between the PC and AC may alter the MT strategy, such as forgoing the use of a balloon guide catheter, which reduces the impact force on the thrombus, allowing more effective retrieval and minimizing the tendency of thrombus fragmentation and distal migration; however, it is rarely used in PCS because of small VA and collateral flow through the contralateral VA [38].
There are significant anatomical variations in the vertebrobasilar circulation and its branches. Asymmetric VAs occur in over two-thirds of the population [39]. Other posterior anomalies include the fenestration of the VA or BA, fetal origins to the posterior cerebral arteries (PCAs), anterior inferior cerebellar artery-posterior inferior cerebellar artery variants, and even the persistence of fetal carotid-basilar anastomoses, which typically regress as the posterior communicating artery develops persistent carotid-vertebrobasilar anastomoses (e.g., persistent trigeminal artery) [13]. Understanding the potential variant anatomy in the PC is imperative when defining the stroke’s etiology and planning EVT.
Several studies have evaluated and compared the outcomes of PC-EVT and AC-EVT. Overall, a lower rate of IVT and a longer interval from stroke onset to recanalization were more frequent in PC-EVT than in AC-EVT. Particularly, ICAS-related occlusions (ICAS-Os) are common in PCS and are associated with relatively poor outcomes and longer and more complicated procedures [23,40]. A meta-analysis showed that PC-EVT was associated with a lower rate of functional independence at 90 days, a higher rate of futile recanalization, mortality, and a lower rate of sICH compared with AC-EVT [19]. Regarding the futile recanalization in PC-EVT, several prognostic factors such as age, baseline NIHSS scores, the number of thrombectomy passes, and intracranial stenting have been identified [20,21].
ICAS-RELATED OCCLUSION IN PC-LVO
ICAS-O, likely due to in situ thrombo-occlusions, is one of the main causes of stroke. Recent studies have reported that ICAS is responsible for 20–40% of patients with a PC-LVO who underwent EVT and approximately 12–30% of all-causes of LVO in East Asia [6]. It is common in the PCS than in the ACS and results in poor clinical outcomes than those resulting from embolic occlusions (without ICAS) [40,41]. ICAS-Os often manifest with a proximal or middle BAO, whereas embolic occlusions result in a distal BAO (Fig. 1A–C) [42]. An occlusion of the proximal and middle BA, which serve most of the pons via perforators, can be related to an extensive ischemia of the pons, leading to fatal conditions such as locked-in syndrome [9]. Additionally, ICAS-O is associated with a longer procedural time and a lower recanalization rate, which may be accountable for the poor outcomes in the PCS [23,40]. Therefore, patients with ICAS-O require a different EVT strategy from that used for patients with an embolic occlusion. Furthermore, optimal PC-EVT strategy should focus on reducing procedural time and restoring perfusion to brainstem in patient with ICAS-O. However, the procedural details for PC-EVT in patients with ICAS-O is not well established.
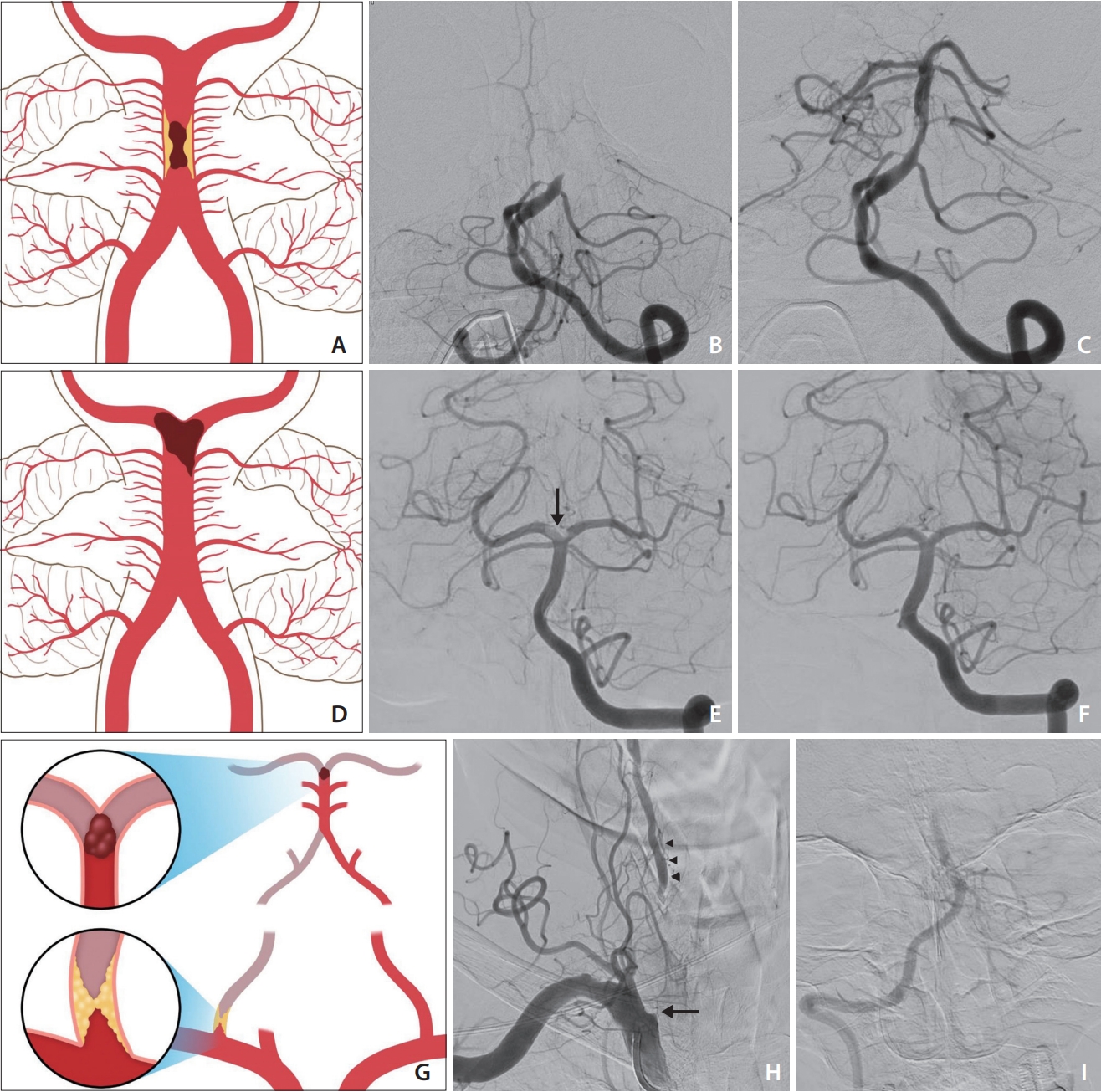
Illustrations and cases of basilar artery occlusion (BAO) according to etiology. (A–C) Middle BAO due to an underlying atherosclerotic stenosis. Initial recanalization was achieved after 2 trials of stent retriever thrombectomy (SRT, not shown). Re-occlusion of the lesion with impaired distal flow was seen on delayed angiogram despite intra-arterial administration tirofiban. Rescue SRT and subsequent balloon angioplasty was performed (not shown). Final angiogram shows recanalization with underlying atherosclerotic stenosis. (D–F) Embolic occlusion of the distal BA. Left vertebral artery (VA) anteroposterior angiogram shows filling defect clot at the tip of the BA involving right posterior cerebral artery origin (arrow). After a single attempt of contact aspiration thrombectomy (not shown), complete recanalization of the BA without residual stenosis is seen. (G–I) Tandem occlusion with a distal occlusion of the BA and a proximal occlusion of the right VA. Right subclavian anteroposterior angiogram shows occlusion at orifice of VA as spike sign (arrow) and distal VA filling (arrowheads) from cervical collateral arteries with impaired antegrade flow. Intracranial anteroposterior image shows occlusion of distal BA.
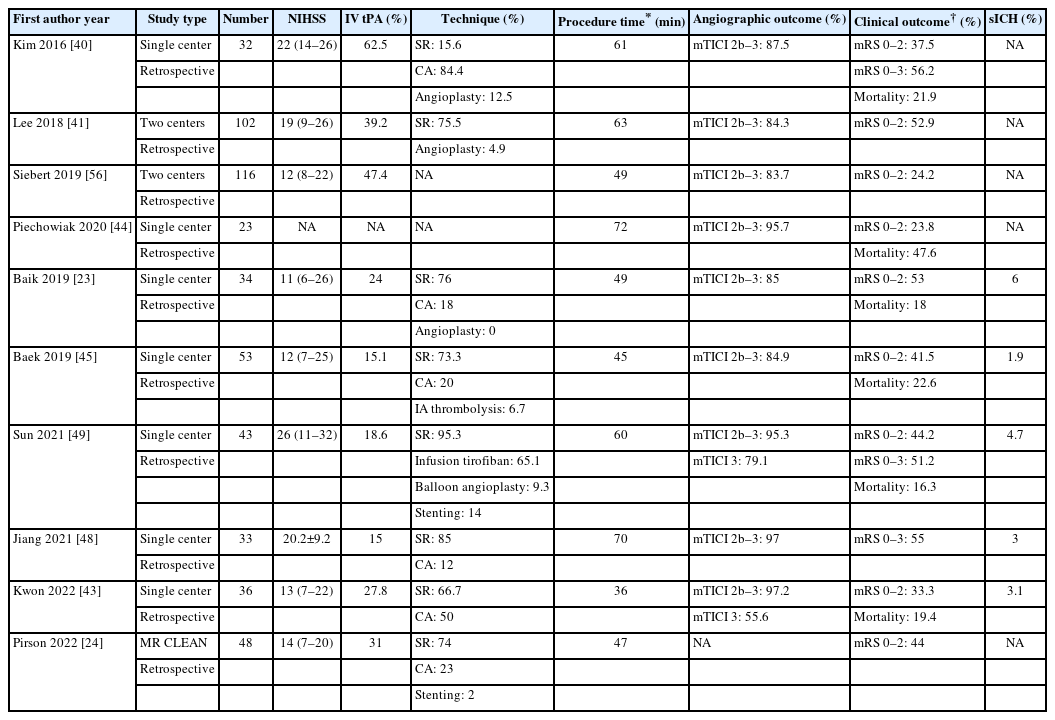
Until recently, few retrospective studies have evaluated the angiographic and clinical outcomes of EVT for ICAS-O in the PC (Table 3) [23,24,40,41,43-49]. Among these studies, the angiographic outcomes were not consistent. Lee et al. [41] found that patients with ICAS-O had a lower rate of successful recanalization. Similarly, Baik et al. [23] found a successful recanalization of 55% in the ICAS group. In contrast, Gao et al. [47] and Kwon et al. [43] reported a high rate of successful recanalization without sICH. These 2 studies have showed a more frequent use of angioplasty than that reported in previous studies [39,49]. Overall, the median procedural time was reported to range from 57–105 minutes. Stent retriever thrombectomy (SRT) is more commonly used as a first-line technique than contact aspiration thrombectomy (CAT) in treating ICAS-O. The rate of favorable outcomes (mRS: 0–2 at 90-day) after MT was 10.5–46.2%.

Studies evaluating outcomes of mechanical thrombectomy for posterior circulation stroke due to intracranial atherosclerosis
SRT may be preferred to aspiration as the first-line technique for primary recanalization of an ICAS-O [50-53]. Although first-line CAT is as effective as SRT in treating embolic LVOs, it is less effective in recanalizing ICAS-related LVOs [53]. Kang et al. [50] reported that front-line SRT is more effective than CAT in achieving primary recanalization with a short procedural time and a less frequently required switching to an alternative thrombectomy technique for an acute ICAS-O. There are several possible explanations for the SRT superiority over CAT in retrieving clots in ICAS-related LVOs. First, most stenoses are tapered and irregularly shaped; thus, it may be impossible to place the tip of a large-bore aspiration catheter in contact with the proximal surface of a clot [50]. This contact is the primary factor in achieving CAT procedural success. In contrast, the SR spans the narrowed section, ensuring full engagement with the entire clot length [50]. Additionally, during SRT, the placement of the SR across the targeted arterial blockage allows for a temporary bypass. This maneuver is not feasible in CAT. The temporary restoration of blood flow can potentially reduce or dissolve the blood clot through natural thrombolysis, which may enable better visualization of the underlying culprit stenosis during SR deployment or after its retrieval [50]. Ultimately, SRT enables the implementation of subsequent rescue therapies, such as intra-arterial administration of a glycoprotein IIb-IIIa inhibitor and the use of a detachable Solitaire stent for permanent stenting [53].
If primary thrombectomy as a frontline technique fails to achieve recanalization, rescue treatments, including switching to another tool (SRT to CAT or vice versa or the simultaneous use of both), intra-arterial thrombolytic infusion, and stenting with or without balloon angioplasty, should be performed as soon as possible for ICAS-Os [51,53,54]. As re-occlusion in ICAS-Os is very frequent and most likely occurs due to platelet activation, severe residual stenosis, or both, rescue treatments should be promptly focused on platelet inhibition and residual stenosis alleviation [53]. Glycoprotein IIb-IIIa inhibitor has been suggested for platelet inhibition in several previous reports. The intra-arterial or intra-venous infusion of a low dose of tirofian (0.5–1.5 mg) is effective in resolving and preventing re-occlusion of ICAS-Os [51,53,54].
Angioplasty and stenting may be considered for PC-LVOs if persistent severe stenosis or re-occlusion occurs after thrombectomy, particularly in cases of poor reperfusion or in a high perceived risk of re-occlusion. However, compared to the internal carotid or middle cerebral arteries, BA has a smaller diameter and more perforating arteries, which can lead to more snow plowing effects during balloon angioplasty or stent placement. Nonetheless, rapid rescue stenting is more frequently required for ICAS-O in the PC [45]. Intracranial stents can be classified as self-expanding stents or balloon-mounted stents. Solitaire AB (Medtronic Neurovascular), Enterprise, and Neuroform EZ/Atlas (Stryker) are aneurysm-bridging intracranial self-expanding stents, which have been widely used off-label in ICAS-Os. Both Wingspan/Gateway (Stryker) and Credo self-expanding stents/NeuroSpeed PTA Balloon Catheter (Acandis GmbH) are approved CE systems [51]. Although the Wingspan self-expanding stents and Gateway Balloon Catheter are the only American Food and Drug Administration-cleared devices for symptomatic ICAS, many neurointerventionists prefer the other systems, especially the Solitaire FR, to the Wingspan [51,53]. It is likely because Wingspan stent requires additional preparation time and more technical demands for delivery, whereas the detachable Solitaire FR, which was already in using for thrombectomy, is simple [51,53-55].
Coronary balloon-mounted stents are commonly used in treating ICAS and are harder to navigate as the addition of a mounted balloon decreases the system flexibility in advancing through a tortuous anatomy [51]. Nevertheless, when technically feasible, the use of balloon-mounted stents is usually easier and faster than the use of self-expanding stents since both balloon angioplasty and deployment are performed as a single step [51,53]. Navigation difficulties can typically be overcome by selecting the shortest possible stent that can treat the lesion and then deliver it through a distal access or intermediate catheter. The Apollo balloon-mounted stents (MicroPort Medical) have been approved for intracranial use in China. However, the number of devices approved for intracranial neurovascular stenting remains limited, and many coronary devices are still routinely used off-label [51].
EMBOLIC OCCLUSION IN PC-LVO
An embolic PC-LVO (e.g., cardioembolism) usually refers to no evidence of a VA steno-occlusion or in situ atherosclerotic thrombosis [23,43]. A embolic occlusion in the BA is usually associated with a distal occlusion, shorter procedural time, higher rate of successful recanalization, and more favorable clinical outcomes than those of other etiologies (Fig. 1D–F) [23,40]. Studies evaluating the procedural and clinical outcomes in PC-LVOs due to embolism are compared in Table 4 [23,24,40,41,43-45,48,49,56]. The median procedural time was reported to be 36–72 minutes. The rate of favorable outcomes (mRS: 0–2 at 90-day) after MT was 23.8–53.0%. The successful recanalization rate was approximately 90%. SRT was mostly used as the first-line MT technique. The frequency of CAT use varied among studies; however, it has been reported as the first-line treatment for embolic BAOs in up to 80% of patients.
Studies evaluating outcomes of mechanical thrombectomy for posterior circulation stroke due to embolic occlusion
Recanalization status and procedural time are relevant factors that affect patient outcomes, regardless of the stroke etiology. Furthermore, it is noteworthy that complete recanalization (TICI: 3) was more beneficial regarding all-cause mortality at day 90 compared with successful recanalization (TICI: ≥2b) [57,58]. Therefore, the MT strategy for fast and excellent recanalization is also required for achieving good outcomes in PC-LVOs due to embolism. This scenario, called the first-pass effect (FPE), which is the achievement of complete or near-complete reperfusion (mTICI: 2c or 3) by a single thrombectomy pass, has been extensively studied for ACS [59,60]. Recently, several studies have shown that FPE was achieved in approximately 20–40% of patients and was associated with improved outcomes in PCS following EVT [61,62]. For PC-LVOs due to embolic occlusion, endovascular strategies should be made to achieve an FPE to maximize the EVT benefit.
The 2 most widely used MT techniques are SRT and CAT. For ACS, 2 RCTs demonstrated comparable efficacy and safety between them as first-line thrombectomy approaches [63,64]. However, a comparison between their effects on PCS remains unclear. Ongoing trials are addressing the first-line technique between SRT vs. CAT for the recanalization of acute BAOs (NCT05320263; PC-ASTER).
Many studies have demonstrated the superiority of the first-line CAT strategy in achieving higher and faster recanalization in acute PCS [61,65-69]. A previous meta-analysis, including 5 observational studies, indicated that first-line CAT can achieve better recanalization results and similar clinical outcomes compared with those achieved by first-line SRT in PCS (mainly BAOs) [68]. Similarly, a recent meta-analysis has suggested that first-line CAT can achieve better recanalization, shorter procedural time, and a higher rate of functional independence compared with those of SRT for patients with acute PCS [69]. Meanwhile, the incidence of hemorrhagic events was similar between the 2 techniques. Additionally, some recent studies have shown that the FPE is related to the CAT technique during EVT in PC-LVOs. Aubertin et al. [61] concluded that CAT, as a first-line strategy, is a strong predictor of FPE in 280 patients with a BAO from the multicenter registry.
First-line CAT has some advantages in treating embolic BAOs. First, the less tortuosity of the VA and the relatively straight course of the BA allow large-bore aspiration catheter navigation [69-71]. Additionally, considering the mechanism of thromboaspiration, the obtuse angle of the distal BA allows good interaction between the catheter and clot [70]. Second, CAT could do with a no-touch technique, as it does not require penetrating the thrombus with a microwire and a microcatheter [72]. It might help to reduce the risk of microwire penetration inside the small thalamic perforating arteries owing to the blunt angle of the basilar bifurcation, which may result in arteries perforation or dissection [73].
The combined technique, SRT+CAT, is widely used for thrombectomy in real-world practice. For patients with ACS, it achieves better recanalization results than those achieved by CAT alone [66,74], especially for carotid T occlusions [75]. A recent retrospective analysis including 2 prospectively maintained stroke registries has shown higher odds of complete recanalization using combined SRT and direct aspiration than those of SRT, direct aspiration alone, or switching techniques [76]. Similarly, Maus et al. [77] compared the combined SRT+CAT technique with CAT in BAOs and observed a higher rate of near complete and complete reperfusion (final mTICI 2c or 3, combined vs. CAT=78% vs. 33%, P=0.006).
The promising results of the combined technique may be explicated by the synergistic effect of the clot trapping between a wedged aspiration catheter that tightly controls the proximal clot surface and an SR that completely engages the whole clot length, permitting full clot extraction while reducing fragmentation and downstream embolization, respectively [76]. Additionally, this technique may reduce antegrade blood flow due to the larger caliber and negative pressure, thus creating an environment similar to flow arrest [28]. Yeo et al. [31] found that BAOs are especially more prone to distal embolization during thrombectomy, particularly with a lack of flow arrest during retrieval and residual antegrade flow from the contralateral VA. Furthermore, flow arrest with balloon guide catheter to reduce distal embolization is not feasible in most cases of PC-EVT due to the dual VA supplies [38]. Therefore, given the PC condition, the combined technique may be an effective thrombectomy option for embolic PC-LVOs.
A dual- or Y-SR may be applied in a distal BAO with refractory clots or large-burden thrombi involving the both PCAs, which may result in a to-and-from movement of the thrombi into the contralateral PCA without an efficient thrombi removal if SR is deployed in only 1 PCA [28]. A case series suggested that the dual SRT may be particularly helpful for refractory clots involving the arterial bifurcation [78]. Li et al. [79] reported that a high successful recanalization rate (100%) could be achieved by the Y-stent rescue thrombectomy technique in 7 patients with refractory basilar terminus occlusion.
TANDEM VERTEBROBASILAR OCCLUSION
Tandem vertebrobasilar occlusion (TVBO) is a pathological mechanism of PCS and is defined as a concomitant VA and BA steno-occlusion (Fig. 1G–I). It was detected in 25–29% of patients with acute PCS [23,44,48,80]. To date, the EVT outcomes of patients with TVBO have been scarcely described in literature (mainly at a single-center) (Table 5) [23,43,44,48,49,56,80-83].
TVBO may harbor procedural difficulties that result in angiographic and clinical outcomes different from those of non-tandem BAOs. When compared with embolic occlusions without tandem lesions, TVBO was more frequently associated with longer procedural time and poorer clinical outcomes (mRS: 0–2 after 3 months; 53% vs. 29%; P=0.05), despite the similar rates of successful recanalization [23]. While another study reported that EVT for TVBO is safe and feasible, with a high rate of good outcomes (53.3%) [44].
For TVBO, various endovascular approaches may be feasible, depending on the dominance of the VAs, vertebrobasilar anatomy, and the type and extent of the occlusive process. Among these approaches are intra-arterial BA thrombolysis or thrombectomy performed through either the contralateral patent VA (clean-road path) or the occluded/affected VA (dirty-road path), without treating the extracranial VA occlusion when the collateral supply is adequate; angioplasty alone or stent-assisted angioplasty followed by treating the intracranial occlusion with thrombolysis or MT (antegrade revascularization technique); and treating the intracranial occlusion with intra-arterial thrombolysis or MT followed by treating the extracranial occlusion (retrograde revascularization technique) [44,80,84]. None of the revascularization strategies (e.g., clean- vs. dirty-road approach, antegrade vs. retrograde technique, or stenting vs. no stenting) proved to be more superior [44,80].
Favoring the clean-road approach is logical, since it is the most rapid, obviates the need for angioplasty, and is a less risky path to achieve the revascularization of the basilar trunk [44,80]. However, accessing the occluded BAs through the contralateral unaffected VA was only achieved in about 25–40% of cases [23,44]. In most cases, the dirty-road pathway via the stenotic or occluded VA was chosen owing to the non-dominant, non-existent, or tortuous contralateral VA [44,80]. Additionally, although the clean-road pathway is easier and faster, the incidence of a recurrent stroke from the non-treated affected VA may be increased. In a case series of 55 patients with TVBO, 2 patients developed short-term re-occlusion of the BA within a week, and both had been treated with a contralateral VA access without angioplasty of the tandem VA lesion [83].
The dirty-road approach may be technically more demanding and potentially riskier than the approach through a clear artery. The identification of the VA origin, microcatheterization of the occluded artery, and reaching the patent lumen are the most challenging steps in an acute proximal VA occlusion [80]. These challenges are analogous to the navigation through internal carotid artery occlusions, theoretical location of the VA origin (facing the internal mammary artery), and detection of the spike sign (Fig. 1H) [85]. In cases of VA occlusions or extremely severe stenosis, pre-dilating the lesions with an angioplasty balloon is essential. Balloon-assisted tracking can be useful for navigating an intermediate catheter (e.g., Sofia or Navien) beyond the occluded VA ostium. However, in cases where the ostium cannot be easily visualized/crossed antegrade initially, it is challenging and prolonging the procedural time [44]. A previous case series regarding TVBO introduced the Synchro Helper to Evaluate via Retrograde Passage an Arterial origin (SHERPA) technique, entailing the passage of a microwire retrograde via the hypoplastic contralateral VA to delineate the vertebral ostium [86].
Both antegrade and retrograde techniques, which are variants of the dirty-road approach, pose significant procedural risks. The antegrade technique first focuses on proximal occlusions, which are stented at an early stage, to secure the VA occlusion. However, this postpones the critical distal thrombectomy step, delaying the reperfusion of the posterior fossa structures [80]. A poorly placed VA stent with a longer protrusion could thwart further intracranial intervention, since navigating the guiding catheter through the stent would be quite difficult [44,80,87].
Owing to these challenges and the need for rapid intracranial reperfusion, the retrograde strategy, postponing the final VA reconstruction until achieving successful intracranial revascularization, is preferred [44,80,81]. The concept behind this strategy is that failed BA revascularization will not require VA reconstruction. The main concern is the risk of VA to BA re-embolization, an event that might be avoided by using balloon guide catheter proximal protection or subsequent suction aspiration [80]. Additionally, the risk of re-embolization when the stent of the ostial VA is implanted can be minimized by positioning a filter-wire in the distal cervical VA after intracranial revascularization prior to stenting [44]. Another concern is the risk of flow arrest. After the passage of guiding/intermediate catheter through the stenotic VA ostium, an occlusion of the residual lumen of the affected VA could occur with consecutive flow arrest [44]. Before planning the retrograde technique, the degree of stenosis as well as the collateral or contralateral VA flows should be checked. Nonetheless, the retrograde technique is preferred because its most important advantage is rapid BA reperfusion. A single-center study of 21 patients with a PC tandem occlusion revealed positive results for the distal-to-proximal strategy [81]. In TVBO, the chosen approach should be tailored to the patient’s anatomic and clinical considerations.
CONCLUSION
Despite the advances in EVT and its success in recent RCTs, futile recanalization and mortality associated with PC-LVOs remain high. The outcomes of EVT are significantly influenced by the underlying pathological mechanism of PC-LVOs. To maximize the efficacy of EVT, the technical strategy should be tailored to the presumed etiology as well as the triage for patient selection. Prospective studies using different stroke etiologies and clinical settings are needed to improve our understanding of the best technical approaches for EVT in patients with a PC-LVO.
Notes
Fund
None.
Ethics Statement
This study was exempted from the review by the institutional ethics committee. This article does not include any information that may identify the person.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: SHB, JYK, and CJ. Analysis and interpretation: SHB, JYK, and CJ. Data collection: SHB, JYK, and CJ. Writing the article: SHB. Critical revision of the article: SHB and CJ. Final approval of the article: SHB, JYK, and CJ, Statistical analysis: SHB. Overall responsibility: SHB, JYK, and CJ.



