Ruptured Medullary Hemangioblastoma Mimicking a Craniocervical Junction Dural Arteriovenous Fistula with a Pseudoaneurysm
Article information
Abstract
Hemangioblastomas (HBMs) are rare vascular tumors commonly located in the posterior fossa of adults. A mid-50s patient presented with sudden unconsciousness. Computed tomography scans revealed acute hemorrhages around the posterior fossa, predominantly in the subarachnoid space. Digital subtraction angiography (DSA) revealed an 8-mm round lesion filled with contrast agent, fed by the C1 segmental artery of the left vertebral artery (VA), showing early venous drainage to the spinal cord and brainstem. Emergent embolization was attempted under suspicion of a ruptured dural arteriovenous fistula, resulting in parent artery occlusion due to feeder selection failure. Follow-up DSA after a month depicted a persistent aneurysm via collaterals from both VAs. Consequently, the decision was made to proceed with surgical intervention, leading to the resection of the lesion, confirming its diagnosis as a HBM through histological examination. This case underscores the potential for misdiagnosis when HBMs with an intratumoral shunt mimic vascular shunt lesions.
INTRODUCTION
Hemangioblastomas (HBMs) are vascular neoplasms commonly associated with von Hippel Lindau disease, though they can also form sporadically [1]. They are the most frequent primary intra-axial tumor in adults, occurring in the posterior fossa [2]. HBMs are varied in appearance, including solid, solid-cystic, or mainly cystic with a small mural, vascularized nidus [3]. While sporadic HBMs typically present as solitary lesions, their marked vascularity makes them easily mistaken for cerebral arteriovenous malformations (AVMs) or even aneurysms [4,5]. Herein, we present a unique case of a ruptured HBM located in the medulla, which mimicked a craniocervical junction dural arteriovenous fistula (AVF) with an aneurysm. This diagnosis was subsequently confirmed post-surgical resection.
CASE REPORT
A patient in their mid-50s presented to a secondary hospital following a sudden loss of consciousness. He had been diagnosed with Crohn’s disease several years prior but was not currently on any medication. Initial computed tomography (CT) showed acute subarachnoid, subdural, and intraventricular hemorrhages around the posterior fossa. A CT angiography highlighted a small, well-enhanced round lesion in proximity to the proximal V4 segment of the left vertebral artery (VA) (Fig. 1A, B). Given the suspicion of a vascular abnormality, a digital subtraction angiography (DSA) was performed and revealed an 8-mm round lesion filled with contrast agent, adjacent to the left VA (Fig. 1C). The lesion appeared to be primarily fed by the C1 segmental artery of the left VA and had an early venous drainage to the spinal and brainstem.
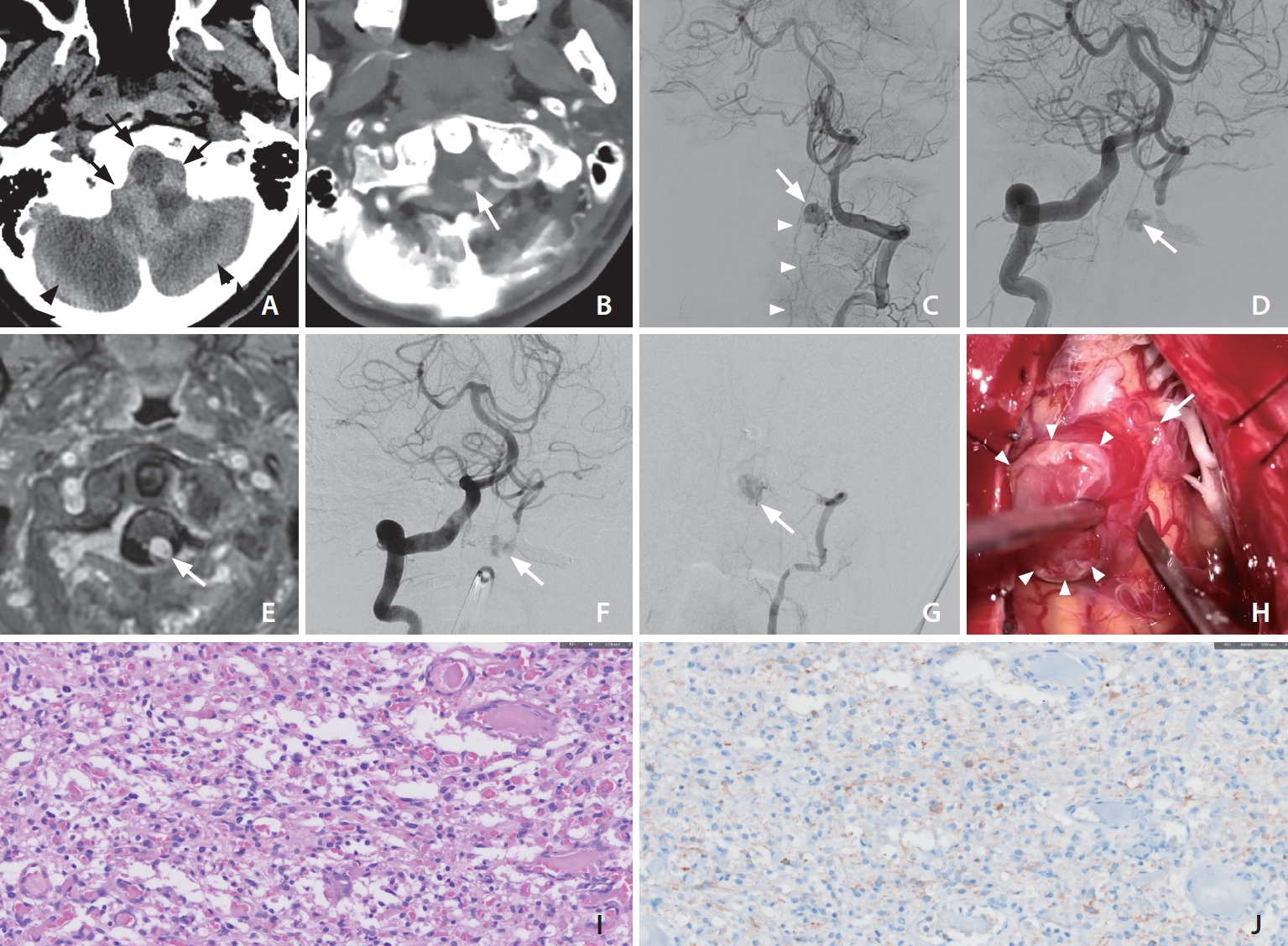
(A) Noncontrast head computed tomography (CT) identified acute subarachnoid (black arrows), and subdural hemorrhages (black arrowheads) around the posterior fossa. (B) CT angiography highlighted a small, contrast-enhancing lesion situated on the dorsolateral aspect of the medulla (white arrow). (C) Left vertebral arteriography depicted a circular contrast-enhanced lesion (arrow) neighboring the left vertebral artery (VA) at the craniocervical junction, with an early draining vein extending downward towards the spinal cord (arrowheads). (D) Even after segmental occlusion of the left VA, the contralateral vertebral arteriography still revealed the same lesion (arrow) through delicate pial collaterals encircling the medulla. (E) After segmental occlusion of the left VA, the aneurysm-resembling lesion also manifested enhancement on the contrast-enhanced T1-weighted image (arrow). (F, G) One-month post-intervention, a follow-up digital subtraction angiography depicted a persistent presence of the aneurysm (arrow) in both VA angiograms. (H) Gross images captured intraoperatively. The mass (arrowheads) was received its blood supply from delicate pial arteries, draining into an arterialized vein (arrow). (I) The nuclei of the mesenchymal cells varied in size (H&E staining, ×400). (J) Inhibin staining was positive, showing the strongly cytoplasmic reactivity (Inhibin-α staining, ×400).
Suspecting a craniocervical junction dural AVF accompanied by a ruptured venous aneurysm, the medical team promptly attempted an emergent embolization to prevent potential rebleeding. However, they encountered difficulties in selecting the feeder artery, leading them to opt for segmental occlusion of the VA instead. Subsequent angiography from the right VA showed the aneurysm remained visible, with pial collateral flow from the right lateral spinal artery (Fig. 1D). Considering the slow blood flow into the suspicious venous aneurysm and the risk to surgery, they chose conservative management over further intervention. In the follow-up magnetic resonance imaging (MRI), a homogeneous enhancing lesion was noted (Fig. 1E). Subsequently, the patient sought a second opinion at our tertiary institution.
One-month post-intervention, a follow-up DSA depicted a persistent presence of the aneurysm in both VA angiograms, indicating recanalization of the previously embolized segment (Fig. 1F, G). Following a comprehensive multidisciplinary consultation, surgical intervention was deemed the best option. During the surgery, the presumed aneurysm was identified as a hypervascular tumor. The surgical team successfully conducted a complete tumor excision without any complications, as highlighted in Fig. 1H. Subsequent histopathological analysis identified the tumor as an HBM, detailed in Fig. 1I, J. After a 2-month hospital stay, the patient exhibited partial recovery, with a modified Rankin Scale score of 4, and was subsequently discharged from our facility.
DISCUSSION
HBMs are uncommon benign tumors, frequent in young to middle-aged adults, with a slight male predisposition [6]. MRI follow-up assessments may contribute to the diagnostic identification of HBMs [7]. On imaging, these tumors usually present as well-defined, homogeneous masses consisting of a cyst with non-enhancing walls and a mural nodule that exhibits vivid enhancement, often accompanied by noticeable serpentine flow voids [8]. Solid HBMs, on the other hand, appear as round-shaped masses that are iso- or hypodense on T1-weighted images and hyperdense on T2-weighted imaging, with a substantial contrast enhancement pattern [9].
HBMs are known to be an extremely rare case of subarachnoid hemorrhage (SAH) [10]. Nonetheless, the occurrence of SAH is documented in infrequent instances attributed to the superficial location of medullary HBMs [11]. Certain authors have put forth postulations regarding pathophysiological mechanisms akin to those observed in AVMs. These postulations suggest that partial transmission of arterial pressure to the venous side, coupled with elevated flow rates, eventually induces structural alterations that culminate in vascular vulnerability [11].
An HBM, manifesting in an extra-axial location, poses challenges in its differentiation from other diseases [12]. Particularly, when it extends outwardly, adjacent to the medullary surface, it can mimic an extra-axial mass, further complicating the diagnostic process. In the spine, it is sometimes seen as an intramedullary as well as an intradural extramedullary mass, and extra- and intradural spinal HBMs are reported in rare cases [13].
It is critical to make a preoperative distinction between solid HBMs and vascular disorders, particularly AVMs and aneurysms. An AVM nidus bears a resemblance to an HBM nodule due to dense collection of vessels [14]. Furthermore, HBMs histologically comprise numerous capillary channels, but an AVM nidus is made up of dysplastic arteries and veins, with the characteristic absence of an intervening capillary bed [15]. Because HBMs are associated with a coexisting feeding artery aneurysm, when hemorrhage is induced by HBMs, a cerebral aneurysm should also be considered [16]. DSA is essential for the differential diagnosis of an HBM, as it enables the identification of the feeding artery and the enlargement of draining veins [17]. As demonstrated in this case, the characteristics on DSA are similar to those of a pseudoaneurysm associated with AVF; thus, a tumor may be mistakenly diagnosed as a pseudoaneurysm prior to surgery. In addition, the aneurysm found in the DSA image of the mid-late arterial phase may be a pseudoaneurysm formed after the tumor ruptured [18].
Surgical resection frequently leads to a curative outcome, and in instances where large lesions are present, preoperative embolization has demonstrated potential benefits in facilitating the procedure [19]. In this case, the size of the tumor was small, and the main feeder was the left posterior spinal artery, but the risk of direct removal may have been very low because trapping was previously performed. Fortunately, since we could immediately ready an alternative procedure, the tumor was successfully resected.
In conclusion, this case report details a patient with a ruptured HBM that was initially misdiagnosed as an AVF with a pseudoaneurysm. It is important to be aware that HBMs exhibiting intratumoral shunts can resemble vascular shunt lesions. Furthermore, it should be noted that HBMs have the potential to manifest as SAHs. This emphasizes the significance of accurate diagnosis and thorough consideration of the various ways HBMs can clinically present, thereby enabling appropriate medical intervention and management strategies.
Notes
Fund
None.
Ethics Statement
A case report or case series containing information on less than 3 patients may not require Institutional Review Board (IRB) of Asan Medical Center approval. This article does not include any information that may identify the person.
Conflicts of Interest
YS has been the Assistant Editor of the Neurointervention since 2018; however, YS has not been involved in the peer reviewer selection, evaluation, or decision process of this article. No potential conflict of interest relevant to this article was reported.
Author Contributions
Concept and design: SP, BK, DHL, and YS. Analysis and interpretation: SP, BK, DHL, and YS. Data collection: SP, JSA, and YS. Writing the article: SP and YS. Critical revision of the article: SP, DHL, and YS. Final approval of the article: YS. Overall responsibility: YS.
