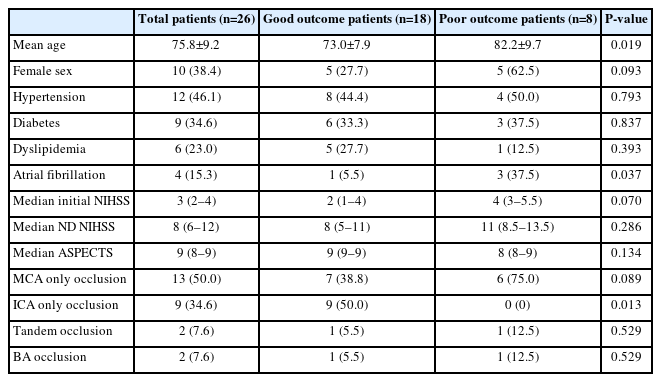
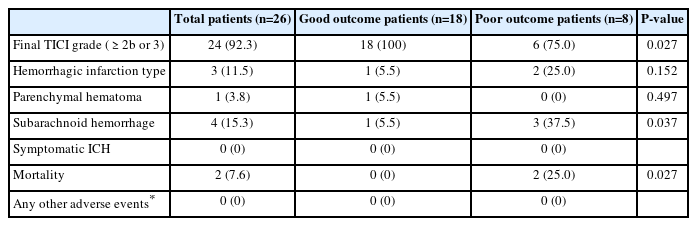
Endovascular Reperfusion Therapy in Minor Stroke with Neurologic Deterioration beyond 24 Hours from Onset
Article information
Abstract
Purpose
Patients with minor stroke (National Institutes of Health Stroke Scale score ≤5) and large vessel occlusion (LVO) often experience neurological deterioration >24 hours after onset. However, the efficacy of endovascular reperfusion therapy in these patients remains unclear. The aim of this study was to determine the efficacy and safety of reperfusion therapy in patients with minor stroke and neurological deterioration >24 hours after onset.
Materials and Methods
Data were retrospectively reviewed from patients between January 2019 and April 2022 who met the following criteria: (1) minor stroke and small definitive ischemic lesions at initial visit, (2) onset to neurological deterioration >24 hours, (3) cortical signs, Alberta Stroke Program Early computed tomography (CT) Score >6 points, and large artery occlusion confirmed by CT angiography at neurological deterioration. Efficacy and safety outcomes were based on final thrombolysis in cerebral infarction (TICI), incidence of symptomatic intracranial hemorrhage (ICH), and mortality. Outcomes were assessed using the modified Rankin Scale (mRS) at 3 months. Good outcome was defined as a mRS of 0, 1, or 2.
Results
Data from 26 patients (38.4% female, mean age 75.8 years) were analyzed; 18 (69.2%) had a good outcome. A final TICI of 2b or 3 was observed in 24 (92.3%) patients. No other adverse events, including dissection, vasospasm or distal embolization, were observed during the procedures. Hemorrhagic events occurred in 8 patients after the procedure; however, there were no symptomatic ICHs. Good prognostic factors were younger age (P=0.062) and carotid stenting (P=0.025).
Conclusion
Endovascular reperfusion therapy performed in selected patients with minor stroke, LVO, and neurological deterioration >24 hours after stroke onset demonstrated favorable outcomes and safety.
INTRODUCTION
Endovascular reperfusion therapy has been shown to improve clinical outcomes in patients with acute ischemic stroke (AIS) [1]. Guidelines recommend considering endovascular reperfusion therapy within 24 hours of onset, but only in cases with good collaterals and penumbra [2].
Neurological deterioration is common in patients with AIS and large artery occlusion. Neurological deterioration may occur after 24 hours and may persist for up to 96 hours [3]. In patients with minor stroke and large artery occlusion, the rate of neurological deterioration is approximately 20% [4]. However, the guidelines for reperfusion therapy in patients with minor stroke and large vessel occlusion (LVO) who experience neurological deterioration beyond 24 hours remain uncertain. Previous studies investigating reperfusion therapy in patients with minor stroke and LVO have reported inconsistent functional outcomes [5-7], and no studies have included patients with neurological deterioration >24 hours after onset. Therefore, this study aimed to determine the efficacy and safety of endovascular reperfusion therapy in patients with minor stroke and LVO who developed neurological deterioration >24 hours after onset.
MATERIALS AND METHODS
This retrospective study investigated patients who underwent endovascular reperfusion therapy for AIS between January 2019 and April 2022. Data from those with AIS, and a modified Rankin Scale (mRS) score of 0 or 1 before the onset of ischemic stroke, were collected and reviewed. Minor stroke was defined as a National Institutes of Health Stroke Scale (NIHSS) score <6, accompanied by symptoms including motor weakness, dysarthria, and decreased sensory function in the absence of a cortical sign, such as aphasia, neglect, hemianopsia, or eyeball deviation. Neurological deterioration was defined as the development of any new neurological symptom(s) with an increase in NIHSS score of ≥4 or the presence of a cortical infarction sign with an increase in NIHSS score of ≥2 [8]. A small definite ischemic lesion refers to an ischemic lesion on brain computed tomography (CT). It is characterized by low density on the scan and is identified as an ischemic core on perfusion imaging, accounting for <1/3 of the area [9,10].
Inclusion criteria for reperfusion therapy in patients with neurological deterioration were as follows: (1) minor stroke and small definite ischemic lesions at the initial visit, (2) onset to neurological deterioration >24 hours, (3) cortical signs, Alberta Stroke Program Early CT Score (ASPECT) >6 points, and large artery occlusion confirmed by CT angiography at the time of neurological deterioration. The occlusion sites included the internal carotid, middle cerebral, and basilar arteries, and sufficient salvageable tissue on CT perfusion and low density of the relevant artery territory, not >1/3 on brain CT at neurological deterioration.
Exclusion criteria were the following: (1) onset to neurological deterioration <24 hours, (2) ASPECT ≤6 points at the time of neurological deterioration, (3) patients with occlusions in the anterior cerebral artery, posterior cerebral artery, and M2 branches of the middle cerebral artery, (4) sufficient salvageable tissues on CT perfusion and low density of relevant artery territory, ≥1/3 on brain CT at neurological deterioration.
In the endovascular reperfusion procedure, access was achieved through the femoral arteries under conscious sedation. Angiography was performed to confirm the presence of occlusion. Mechanical thrombectomy was performed using a combined technique involving a stent retriever (SolitaireTM; Medtronic) and an aspiration catheter (5Fr SofiaTM; MicroVention) at the occlusion site. After thrombectomy, in cases with re-occlusion or stenosis due to atherosclerosis confirmed by delayed angiography, balloon angioplasty and stenting is applied [11]. Balloon angioplasty (GatewayTM; Stryker) was performed followed by stent placement (EnterpriseTM 2; Cerenovus). After stenting, tirofiban was administered both intra-arterially and intravenously in cases with in situ thrombus [12].
After the procedure, the patient was immediately transferred to the stroke or intensive care unit for a minimum of 48 hours, where vital signs and neurological symptoms were continuously monitored. If there was no worsening of the clinical condition, brain magnetic resonance imaging (MRI) was performed within a mean (±standard deviation [SD]) 24±8 hours. If there was no evidence of significant hemorrhagic transformation, dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) with high-intensity statins (atorvastatin 40 mg or rosuvastatin 20 mg) was initiated via oral administration.
Patient characteristics and clinical information, including age, sex, medical history, initial NIHSS score, neurological deterioration NIHSS score, ASPECT score, and occluded artery, were recorded. Endovascular reperfusion procedure data, including the last known mean normal time to arterial puncture, procedure methods, stent insertion, and stent insertion site, were collected. Brain MRI was used for post-procedure imaging assessment.
Safety evaluation included peri-procedure adverse events, hemorrhagic infarction, parenchymal hematoma, subarachnoid hemorrhage, and symptomatic intracranial hemorrhage (ICH). Hemorrhagic transformation was defined according to the European Cooperative Acute Stroke Study classification [13], which was divided into hemorrhagic infarction and parenchymal hematoma. There are 2 types of hemorrhagic infarction: type 1, with small petechial bleeding; and type 2, with a more widespread bleeding pattern. Parenchymal hematoma is also split into 2 types: type 1, with hematoma size <30% of the infarcted brain area; and type 2, with hematoma size ≥30% or extending beyond the region of cerebral infarction. Symptomatic ICH was defined as an increase in NIHSS score by ≥4 from pre-procedure or any new symptom(s) [14]. Good outcomes were defined as mRS scores of 0, 1, or 2.
Statistical analysis was performed using SPSS version 22 (IBM Co.). Continuous variables are expressed as mean±SD and median with interquartile range; discrete variables are expressed as number and percentage. An independent t-test was used to compare the means of the 2 groups. Pearson χ2 and Fisher’s exact tests were used to compare discrete variables.
RESULTS
During the study period, 26 patients were selected from a total of 578 patients who met the inclusion criteria. Good outcomes were achieved in 18 patients (69%). The mean age of this group was approximately 10 years younger (P=0.019). The median ASPECT score for patients who underwent the procedure was 9 points (Table 1).
The mean (±SD) last known normal time to arterial puncture time was 59.9 hours. There was no significant difference between those with good and poor outcomes. Emergency stenting was performed after mechanical thrombectomy in 20 of the 26 patients. Of the 20 patients with stent insertion, 17 had a favorable outcome. Of the stenting procedures, 11 patients underwent intracranial stent insertion for M1, basilar artery, and tandem occlusion, and 9 patients underwent carotid stent insertion. The prognosis was better in patients with carotid stent insertion than with intracranial stent insertion. Furthermore, all patients who underwent thrombectomy without stenting had an unfavorable prognosis (Table 2).
Twenty-four of the 26 patients achieved a final thrombolysis in cerebral infarction grade of 2b or 3. Only 2 patients had a fatal outcome, 1 due to reocclusion, and 1 due to sepsis. No other adverse events, such as dissection, vasospasm or distal embolization occurred during the procedure. Post-procedure hemorrhagic events occurred in 8 patients, but none developed symptomatic ICH (Table 3).
The most common imaging finding in patients with stenting was the presence of in situ atherosclerotic stenosis as demonstrated by digital subtraction angiography (Fig. 1A–C). In 10 of 26 patients, a comparison of diffusion-weighted imaging results before and after the onset of neurological deterioration was possible. There were no territorial infarctions beyond the dot or scattered small lesions (Fig. 1D, E).
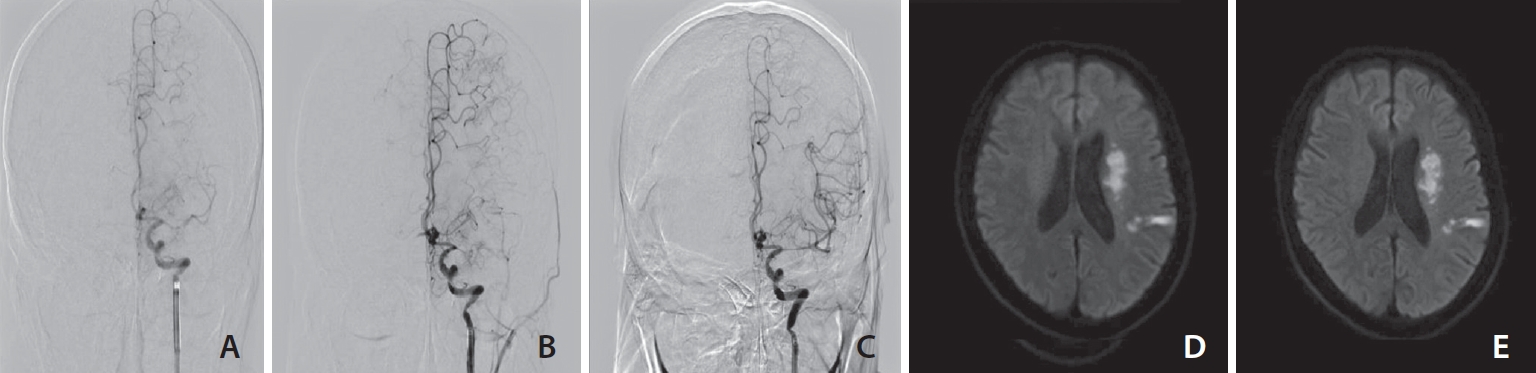
Endovascular recanalization therapy and brain diffusion-weighted imaging before and after 24 hours of the procedure. (A) In digital subtraction angiography performed 83.9 hours after occurrence, left MCA proximal site occlusion and decreased filling on distal flow. (B) After 1 thrombectomy, a partially recanalized, but left MCA mid portion diffuse severe atherosclerotic stenosis and decreased distal flow were detected. (C) Stent insertion was carried out, full recanalization and TICI grade 3 of distal flow were identified. (D) Diffusion restriction of left periventricular area and temporo-parietal lobe at initial. (E) After 24 hours of the procedure, there was no significant difference compared with the previous ischemic lesion. MCA, middle cerebral artery; TICI, thrombolysis in cerebral infarction.
DISCUSSION
In our study, endovascular reperfusion therapy performed in selected patients with minor stroke, LVO, and neurological deterioration >24 hours after onset resulted in favorable functional outcomes. Additionally, there were no serious complications resulting in symptomatic ICH or direct fatal outcomes due to endovascular reperfusion therapy. Patients who underwent carotid stenting had better functional outcomes than those who did not. Several studies have reported on endovascular reperfusion therapy in patients with minor stroke and LVO [4,5]. Furthermore, previous studies have predominantly focused on patients who underwent reperfusion therapy, including thrombectomy within 24 hours [15,16], and on those who underwent endovascular reperfusion therapy >24 hours after stroke onset. However, there is no consensus on the outcomes of mechanical thrombectomy. Some studies suggest a lower incidence of favorable outcomes, while others report similar outcomes [14,17].
Guidelines recommend that endovascular reperfusion therapy be considered within 24 hours of onset. We conducted a study specifically focusing on endovascular reperfusion therapy beyond 24 hours after stroke onset due to the limited data available in this regard. Thus, despite the limited sample size, our research data provide additional evidence to support the positive impact of thrombectomy performed >24 hours, as previously reported in other studies [14].
The majority of patients who underwent emergent stenting were found to have underlying stenosis confirmed by imaging. In cases where such pathology exists, mechanical thrombectomy alone is insufficient, and emergent stenting becomes necessary, as indicated by previous studies [18]. In our study, we also performed emergent stenting in patients with large-artery atherosclerosis, yielding favorable outcomes. This may be attributed to the presence of well-established compensatory collateral circulation and the maintenance of penumbra, which could be associated with atherosclerosis and underlying stenosis of the relevant vessels [19,20].
Our study had several limitations, the first of which was its retrospective design and the inherent risk of selection bias because only patients with good collateral circulation or ASPECT scores underwent the procedure. Second, the number of patients included was small; therefore, it is difficult to generalize these results. Third, the evaluation of salvageable tissue was based on visual assessment, such as ASPECT, rather than using objective measurement software.
CONCLUSION
Endovascular reperfusion therapy performed in patients with minor stroke and LVO resulting in neurological deterioration >24 hours after onset demonstrated favorable outcomes without symptomatic ICH or other adverse events. Thus, well-designed, larger-scale studies aimed to determine the efficacy and safety of endovascular reperfusion therapy in this patient population are warranted.
Notes
Fund
None.
Ethics Statement
Our study has received the Institutional Review Board of the School of Medicine, Chosun University approval (IRB FILE No: CHONSUN 2023-09-017). This article does not include any information that may identify the patient.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: SHA. Analysis and interpretation: MAL and BWH. Data collection: MAL, BWH, SWH, JHK, and HSK. Writing the article: MAL. Critical revision of the article: SHA. Final approval of the article: SHA. Statistical analysis: MAL. Overall responsibility: SHA.