A Case of Severe Delayed Vasospasm after Clipping Surgery for an Unruptured Intracranial Aneurysm
Article information
Abstract
Delayed ischemic stroke associated with intractable vasospasm after clipping of unruptured intracranial aneurysms (UIAs) has been rarely reported. We report a patient with delayed ischemic stroke associated with intractable vasospasm following UIA clipping. A middle-aged female underwent surgery for unruptured middle cerebral artery bifurcation aneurysms. The patient tolerated the neurosurgical procedure well. Seven days postoperatively, the headache was unbearable; a postcraniotomy headache persisted and abruptly presented with global aphasia and right-sided hemiplegia after a nap. Emergency digital subtraction angiography showed severe luminal narrowing with segmental vasoconstriction, consistent with severe vasospasm. The patient’s neurological deficit improved after chemical angioplasty. Neurosurgeons should pay close attention to this treatable/preventive entity after neurological deterioration following UIA clipping, even in patients without subarachnoid hemorrhage.
INTRODUCTION
With the recent development of neuroimaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), the detection of unruptured intracranial aneurysms (UIAs) is rapidly increasing [1]. The prevalence of UIAs is estimated to be 3.2%, and UIAs are the leading cause of nontraumatic subarachnoid hemorrhages (SAHs). The fatality rate of patients after rupture of intracranial aneurysms has been estimated to be approximately 50% [2]. Thus, depending on the location, size, and morphology of the UIA, implementing the best strategies to prevent grave SAH is crucial [3]. Although limitations to the interventional approach have progressively decreased due to increasing expertise and some technical refinements, aneurysmal clipping is still regarded as the best therapeutic option for middle cerebral artery (MCA) bifurcation aneurysms among neurosurgeons [4]. Also, surgical clipping for UIA treatment has shown greater advantages in terms of safety and efficacy due to the specific anatomical characteristics of the MCA bifurcation complex [5]. Moreover, the risk of perioperative complications has significantly decreased compared to that in the past due to the development of microscopic surgery and advanced neuro-anesthesia techniques [6]. Importantly, treating UIAs rarely causes ischemic brain damage associated with vasospasm, so managing patients with UIA has led to much better clinical outcomes than treating patients after rupture. Nevertheless, ischemic events following unpredictable delayed vasospasm after clipping have been reported in UIA patients [7-20]. Here, we report a case in which delayed vasospasm and ischemic stroke accompanied by intractable headache occurred 1 week after the successful clipping of an unruptured MCA bifurcation aneurysm.
CASE REPORT
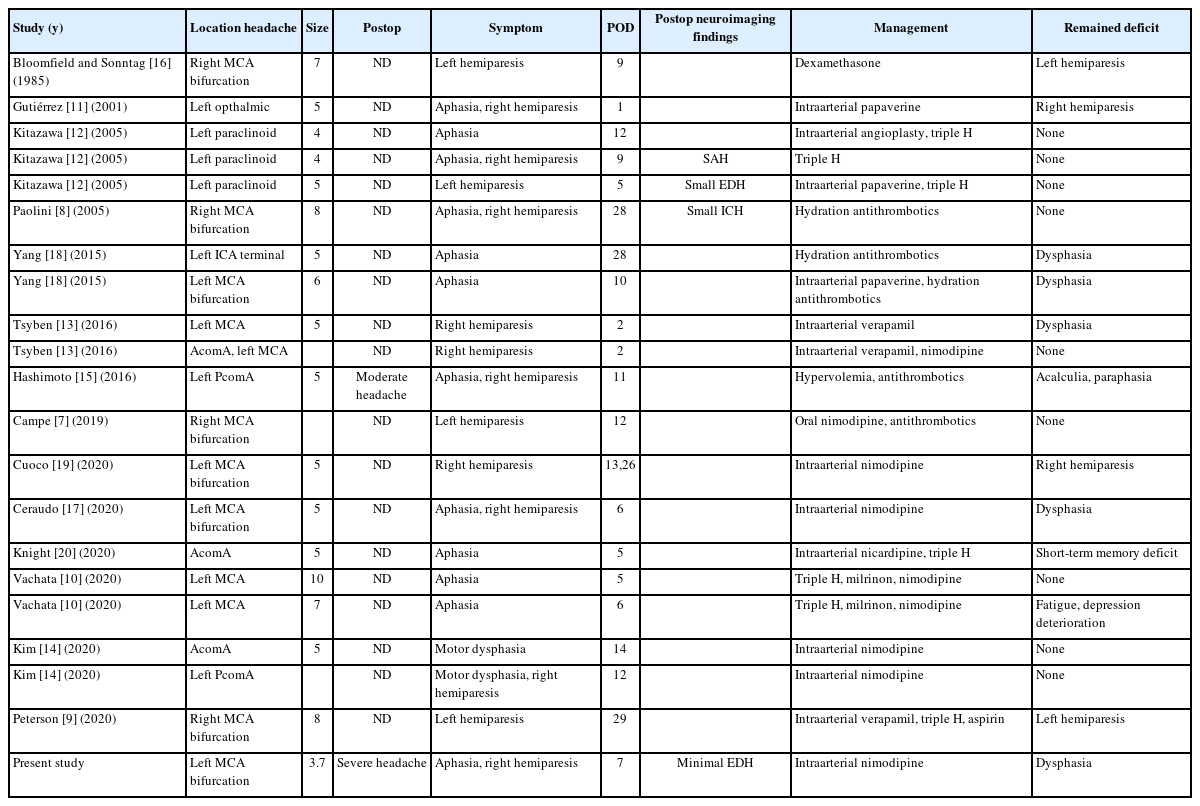
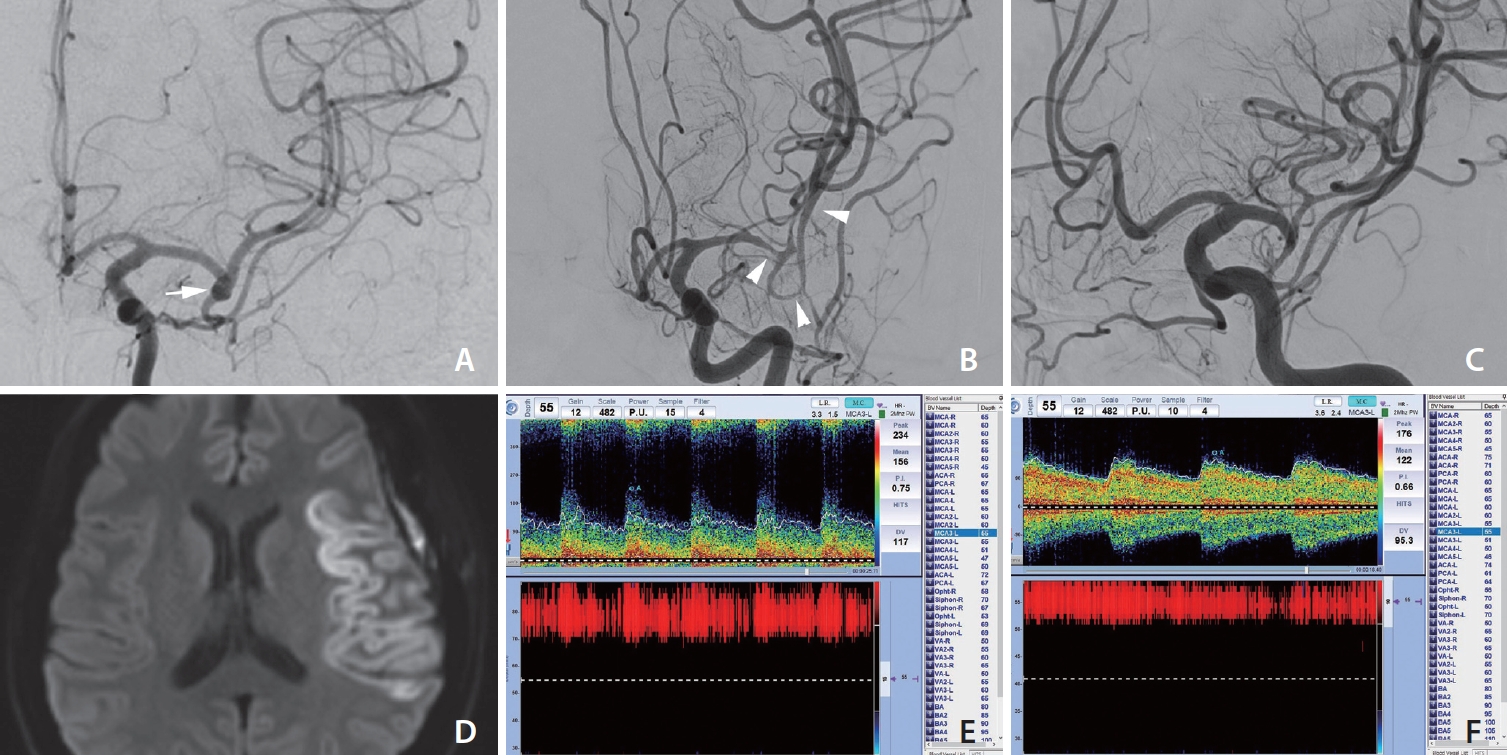
A middle-aged patient without any comorbidities received a brain MRI at the neurology department following an episodic headache. The patient denied any other neurological symptoms, and the patient neurological examination was normal. Initial MR angiography and digital subtraction angiography (DSA) demonstrated an incidental 3.7×2.2 mm aneurysm with a bleb in the dome arising from left MCA bifurcation (Fig. 1A). The patient opted to visit another hospital, and after a discussion with the neurosurgeon, the patient decided to undergo elective clipping of the left MCA bifurcation aneurysm. Postoperatively, the patient was transferred to the neurointensive care unit and briefly experienced a single seizure episode. The patient tolerated the neurosurgery well and was discharged on postoperative day 3 with residual headache. However, at 7 days postoperative, the patient was admitted to the local hospital to manage an unbearable, sustained postcraniotomy headache without any neurological deficits. On day 3 after admission, the headache was well managed with a painkiller, and the patient presented with global aphasia and right-sided hemiplegia after a nap. Neuroimaging studies with pre-enhanced CT and diffusion-weighted brain images revealed severe left MCA territory infarction (Fig. 2A–C). Thus, the patient was transferred to a comprehensive stroke center, and a novel perfusion MRI revealed decreased cerebral flow throughout the left MCA region.
Digital subtraction angiography (DSA) findings and transcranial Doppler changes before and after treatment. (A) DSA image showing a normal vascular diameter and normal vascular appearance at the initial exam. Contrast stagnation was observed at the left middle cerebral artery (MCA) bifurcation, suggesting an unruptured saccular aneurysm (white arrow). (B) Severe luminal narrowing around the MCA bifurcation (white arrowheads) and overdilated M2–3 segments were observed on DSA at the first neurologic deterioration. (C) Marked resolution of vasospasm was shown after intraarterial nimodipine. (D) Diffusion restriction at the left MCA territory was confirmed at the transferred hospital. (E) The mean flow velocity was increased (156 cm/s, 55 mm) and the Lindegaard ratio was 5, which are indicative of moderate to severe vasospasm. (F) Improvement of vasospasm was shown by transcranial Doppler monitoring after 2 nimodipine injections.
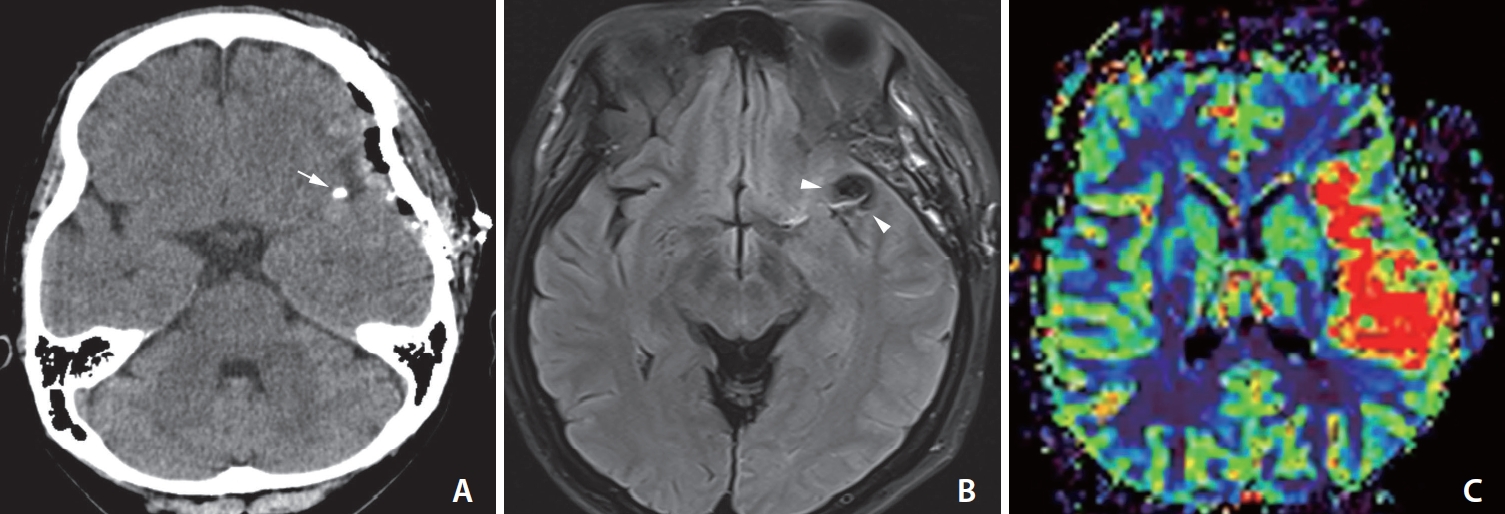
Initial neuroimages of the patient at the ischemic symptom onset. (A) Computed tomography scan demonstrates the successful clipping of the left middle cerebral artery (MCA) bifurcation aneurysm without significant hyperacute stroke. Very bright, high signal intensity at the left Sylvian fissure regarding the metal clip artifact was observed (white arrow). (B) Some suspicious hyperintense vessel signs on the fluid attenuated inversion recovery image suggested that ischemic changes (white arrowheads) are noted in the left Sylvian region and temporal lobe. (C) Magnetic resonance perfusion showing marked increased mean transit time in the left MCA distribution.
Next, an emergent DSA was performed, and the initial angiography revealed severe luminal narrowing with segmental vasoconstriction, which suggested severe vasospasm (Fig. 1B). Intraarterial nimodipine was instilled after internal carotid artery selection (5 mg twice for the initial 2 days) with angiographic control showing a marked improvement in the caliber of these segments. To optimize cerebral blood flow, systemic norepinephrine (target blood pressure 160–200 mmHg) was added. The patient was admitted to the neurointensive care unit and received volume expansion and permissive hypertension treatment. One day later, a repeat DSA showed mild improvement in the vasospasm in the same vessel. The patient received an additional 5mg of intraarterial nimodipine again with marked improvement in vasospasm (Fig. 1C). Transcranial Doppler monitoring of the MCA vasospasm revealed improvements in the mean flow velocity and Lindegaard ratio after intraarterial treatment (Fig. 1E, F). Unfortunately, the patient MRI showed an infarction involving the left MCA region (Fig. 1D). The patient’s neurological status gradually improved after chemical angioplasty. The patient was able to live independently, but prominent motor dysphasia was still present until hospital discharge.
DISCUSSION
Cerebral vasospasm, which is relatively uncommon, has been reported to occur after the clipping of a UIA [7-20]. Here, we reported a case of a middle-aged female who suffered from an ischemic stroke due to severe vasospasm following successful treatment of an unruptured MCA bifurcation aneurysm. The pathomechanism of the unexpected severe vasospasm remains unclear. Because the etiology of cerebral vasospasm can vary, the underlying mechanisms are likely multifactorial. Even the exact mechanism of vasospasm following SAH is still not completely understood. Several potential mechanisms have been identified that may regulate or reflect the pathogenic alterations of cerebral vessels in vasospasm, such as (1) dysregulation of cerebral vascular tone, (2) sympathetic overactivity, (3) endothelial dysfunction, (4) excessive oxidative stress, and (5) blood-brain barrier disruption [21,22]. The breakdown of products from injured erythrocytes, such as oxyhemoglobin in the aneurysmal sac, has been considered an inducer of vasospasm [21]. Mechanical manipulation around the major branches of the MCA may lead to impaired cerebrovascular reactivity, which induces a vasospastic response. The trigeminal-cerebrovascular system has also been regarded as a potential risk factor for cerebral vasospasm. The activation of trigeminal sensory nerves around the circle of Willis releases vasoactive molecules, resulting in cerebral vasodilation to maintain a normal vessel diameter [9,21]. Cerebral vasospasm does not occur exclusively in SAH and can be observed in patients with subdural hematoma following hematoma evacuation, intracranial tumor resection, or traumatic brain injury [23].
Postcraniotomy headaches are a common symptom after surgery, and most of the time they can be completely resolved without any complications [24]. Thus, most of the case reports did not emphasize symptoms of vasospasm before ischemic events. However, even though severe vasospasm rarely occurs in the neurosurgical field, severe vasospasm must be suspected in patients with sustained, intractable headaches after clipping surgery. We analyzed 17 cases that reported ischemic events associated with vasospasm that occurred after UIA clipping surgery (Table 1). Fourteen of the 17 cases were females, and most were middle-aged females (40–60 y). Postoperative pain was found to be more common in females, who had higher pain scores [25]. Females with SAH have a higher risk of radiographic vasospasm, clinical deterioration, and cerebral infarction [26]. Interestingly, most patients who experienced ischemic events after elective clipping surgery were middle-aged females [14]. Thus, assessing whether other sex-specific factors could contribute to these findings should be highlighted.
Nevertheless, there is no standard protocol for management. Previous studies have reported successful treatments with nimodipine or verapamil, mostly with favorable outcomes after managing the spasm [7-20].
In conclusion, sustained intractable headaches after UIA treatment can be a severe premonitory sign of cerebral vasospasm. Although outcomes are favorable, the consequences can include a risk of permanent neurologic deficit. Early awareness and proper management can prevent undesirable results.
Notes
Fund
None.
Ethics Statement
Ethical approval was granted in accordance with national requirements, and the need for written informed consent was waived (Institutional Review Board of Jeju National University Hospital). We anonymized patient information, such as sex and age, in the case section to prevent the identification of individuals.
Conflicts of Interest
YS has been the assistant editor of Neurointervention since 2018. However, he/she has not been involved in the peer reviewer selection, evaluation, or decision process of this article. No potential conflict of interest relevant to this article was reported. No other authors have any conflicts of interest to disclose.
Author Contributions
Concept and design: JGK, YS, and JKR. Analysis and interpretation: JGK, JJS, and CHK. Data collection: JGK, YS, and JJS. Writing the article: JGK. Critical revision of the article: JKR. Final approval of the article: JKR. Overall responsibility: JKR.