Improving Forward Infusion Pressure during Brain Tumor Embolization with the Double Catheter and Coil Technique
Article information
Abstract
Endovascular embolization or embosurgery of brain tumors can be used to reduce neoplasm vascularity prior to surgical resection. Two challenges with embosurgery relate to insufficient perfusion pressure into the tumor and inadvertent escape of infused agents into parenchymal branches of the adjacent brain. This report describes a multi-catheter and coil technique to improve tumor perfusion and prevent reflux into normal branches.
Pre-resection embolization or embosurgery of brain tumors has become a staple adjunct to surgical tumor extirpation with the primary objectives: to reduce intraoperative blood loss by selectively occluding deep, inaccessible arterial tumoral feeders which in turn will facilitate definitive resection by improving visualization in during resection [1]. The tumor devascularization is achieved by microcatheter directed arterial injection of one or more variety of agents, including PVA particulates (polyvinyl alcohol and acrylic microspheres), sclerosing liquid agents (ethanol, adhesive and non-adhesive glue such as n-butyl cyanoacrylate or ethylene vinyl alcohol copolymer, respectively). Tumoral parent vessel sacrifice with coils is also an option with embosurgery.
These endovascular techniques induce variable ischemic necrosis of tumor, with softening of the mass, mitigating its stringent compression on nearby neural structures, and enhancing a more safe and efficacious resection [12]. In order to maximize tumoral necrosis, a multi-catheter and coil technique was utilized and described in this case report to improve tumor penetration while preventing undesirable embolic agent migration into the normal adjacent brain.
CASE REPORT AND DESCRIPTION OF THE TECHNIQUE
A 64-year-old male presented with 1-year duration of worsening dysphagia, neck pain and stiffness. Physical exam was remarkable for right upper extremity dysmetria, mild weakness and diminished sensation, as well as left deviation of the uvula and palate. MRI of the craniocervical junction showed a 4-cm right anterior foramen magnum mass extending from the lower clivus to the C1/2 junction, most consistent with meningioma (Fig. 1). It encased the right vertebral artery and encroached upon the left vertebral artery. The medulla and cord were displaced dorsally. Angiography confirmed a tumoral blush characteristic of meningioma. Major supply to the mass included the C2 and C3 odontoid arcade branches of the dominant right vertebral artery.
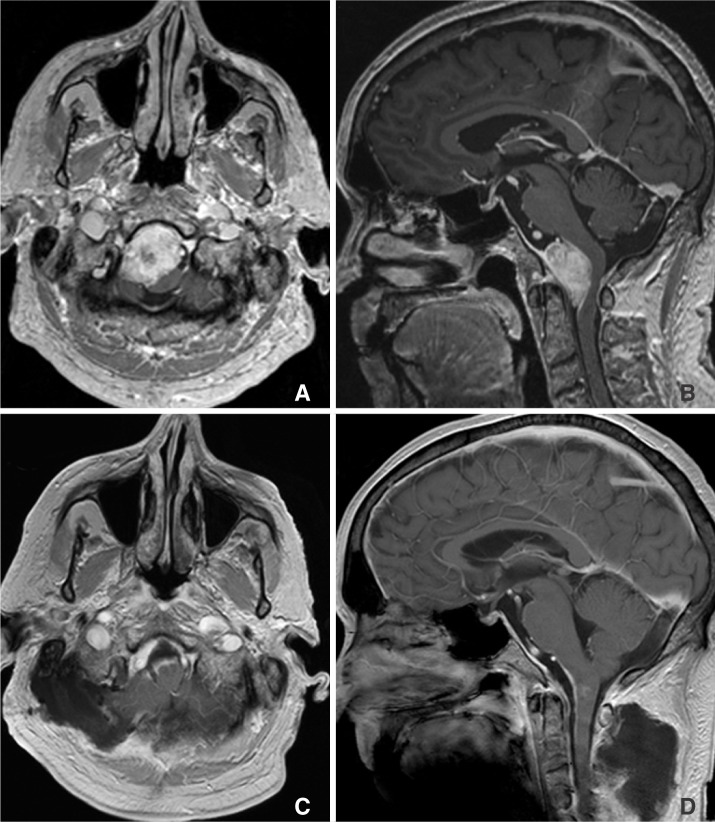
Baseline axial (A) and sagittal (B), and post-resection axial (C) and sagittal (D) T1 enhanced MR images of the foramen magnum meningioma.
Superselective angiography of the slightly larger C2 branch was done using the Excelsior SL-10 microcatheter (Stryker Neurovascular; Fremont, CA, USA). This showed retrograde opacification of the right vertebral artery via the right C3 as well as retrograde filling of the vertebral artery via the C2 branch (Fig. 2). This catheter was left in place while a second Excelsior SL-10 was placed into the right C3 branch. With two microcatheters now positioned-one in each pedicle, angiography showed that the C3 microcatheter was occlusive and prevented retrograde contrast reflux when either the C2 or C3 catheters were injected (Fig. 3). Because injection of the C2 microcatheter demonstrated reflux around itself back into the vertebral artery, this route could not be used for particle embolization. On the other hand, in order to safely embolize via the C3 microcatheter, a 1.5×30 mm Target Helical UltraSoft platinum coil (Stryker Neurovascular; Fremont, CA, USA) was placed at the tip of the C2 microcatheter to prevent the passage of particles to the vertebral artery and ensuring increased embolic infusion with the C3 catheter. Protection of the C2 branch microcatheter by coiling was confirmed with C3 branch angiography (Fig. 4). Flow controlled embolization via the right C3 tumoral branch with a dilute suspension of 150-250 m Contour PVA particles (Boston Scientif ic; Fremont, CA, USA) was then completed. Next, the C2 branch coil was detached. Finally, a 1×20 mm Target Helical UltraSoft platinum coil was placed and detached in the C3 branch to complete the right vertebral artery territory embosurgery.

Native (A) and subtracted (B) angiograms of the right vertebral C2 (black arrow) and the C3 branch tumoral pedicles (white arrows). Both pedicles supplied the enhancing mass, and both demonstrated retrograde reflux into the vertebral artery when either adjacent branch was injected.
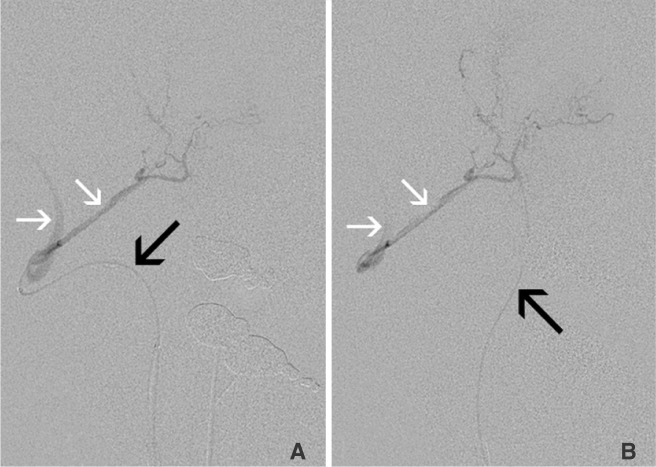
Subtracted angiograms via microcatheters in the right vertebral C2 (A) and C3 (B) branch tumoral pedicles. Reflux (white arrows) along the C2 microcatheter back into the vertebral artery occurs during injection of either the C2 (A, black arrow) or C3 (B, black arrow) microcatheters. The non-injection C3 microcatheter (A) and the C2 microcatheter (B) are not well visualized due to subtraction.
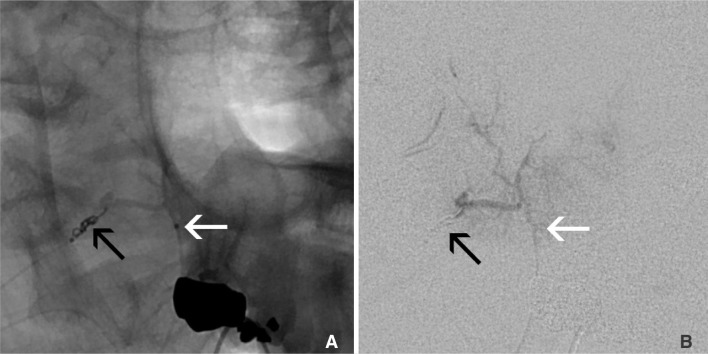
With a catheter in the C3 branch (A, B, white arrow), PVA embolization was safely performed because of the coil protection provided by the double catheter technique in the adjacent C2 branch (A, B, black arrow).
Two days following embosurgery, the patient underwent resection of the mass via an extreme right lateral craniectomy approach with C1/2 laminectomies. Estimated blood loss was 1500 mL. Pathology demonstrated a WHO-Grade I meningioma. Postoperative and 34 month follow-up MRI brain demonstrated an unchanged, small tumoral remnant (Fig. 1).
DISCUSSION
Meningiomas present predominantly in middle-aged to elderly females, accounting for about a third of all primary tumors diagnosed in the brain [3]. While 90% of these tumors are benign, some of these tumors may impinge on vital intracranial structures that often necessitate open surgical resection [4]. Advances in microsurgical technique and complex skull base approaches have allowed neurosurgeons to offer treatment to more patients with meningiomas, but resecting these tumors from eloquent or deep brain tissue can remain challenging [5]. However, innovation in neuroendovascular surgery with regards to embolic materials and microcatheter technology has expanded the window of opportunity for resecting the more difficult neoplasms [6].
Embosurgery of meningiomas has gained prominence as an essential adjunct to surgical resection because it reduces intraoperative blood which in turn facilitates definitive resection through improved surgical visualization [1]. Resection is typically performed 1 or few days afterwards, permitting tumor necrosis to take place. If delayed beyond this time frame, swelling of the tumor may occur along with neovascularization [78]. While embosurgery may overall facilitate the operative tumoral resection, and may diminish the complications while improving outcomes when compared to resection alone, the application of embolization is subject to debate, requiring balanced consideration of its pros and cons in given the particularities of each case [19]. Key components of a successful embosurgery include a comprehensive and thorough knowledge of the patient's unique neurovascular anatomy, venous outflow patterns, dural sinus involvement, anatomical variation, and regional anastomoses to avoid devastating unintended embolization of critical non-tumoral cerebrovascular territories [10]. Prognostic factors favoring neoplasm embolization include: hypervascularity, large size and arterially accessible location [8].
Embolization can be done by with either a transarterial or direct tumoral puncture approach. A transarterial route is most commonly utilized, in which a microcatheter is maneuvered into the tumoral feeding vessel. However, this method may be challenging if vascular anatomy involves tortuous or small caliber vessels, which can thwart all attempts to gain the necessary superselective microcatheter position. Also, the presence of local collaterals as in the case report may pose a hazardous risk for refluxing embolic material into the normal neighboring branches. Furthermore, the injection of only a single feeding artery may prove inadequate in devascularizing the tumor, necessitating additional selective catheterization. These issues lengthen the overall operation time and increase the risk of nontarget arterial embolization [1112].
Direct puncture embolization has been advocated as an alternative to address these problems. However, this technique requires trephination for neoplasms that do not breach the cranium. Utilizing an 18-25 gauge needle, a liquid embolic agent is directly injected into the tumor bed under fluoroscopic guidance. While this achieves a higher concentration of tumoral embolic agent and avoids the need for endovascular catheterization, it has an increased likelihood of inadvertent transtumoral embolization of venous structures due to shorter distance to travel before reaching the venous system [1314]. Also, direct tumoral injection has potential to reflux the arterial feeders with possible inadvertent migration to normal structures. Overall, the choice of either approach is determined by factors such as location, number of arterial feeders, vascular access/tortuosity, individual tumor characteristics, presenting symptoms, patient age and condition.
In this case report, a transarterial approach with two microcatheters was required to permit a safe embolization of a foramen magnum meningioma. Larger PVA particles were used to penetrate and embolize the tumoral bed arterioles. Devascularization was completed by coiling off these feeder vessels. Simple occlusion of the proximal feeding arteries with coils alone has been shown to result in less effective devascularization; Also, larger PVA particles (150-250 µm) versus smaller ones (45-150 µm) decreases the risk of unintentional embolization to normal, nontumoral anastomosing intracranial branches [61315]. After particulate embolization, placing detachable coils in the feeding arteries serves to maximize the occlusive effect, to provide a radiopaque landmark, and to prevent retrograde migration of embolic particles. Before coiling, insuff icient tumor perfusion was observed due to the presence of an adjacent collateral vessel. After forming a coil basket in the collateral vessel with a double catheter technique, targeted tumoral flow was observed. It follows that placement of coils allowed for adequate buildup of forward perfusion pressure to ease undisrupted anterograde blood flow in supplying branches, and allowed for greater perfusion and penetration of the tumor by the embolic material. Maintenance of continuous and steady flow is vital because it ensures that embolizing material is carried to target vasculature while migration of embolic particles to normal vasculature is prevented. As in this case, if the collateral vasculature is not protected, as with the double catheter technique, flow arrest can lead to inadvertent migration of particles through nontarget arterial vessels [810].
This purported catheter and coil technique may also prove to be of utility to the local delivery of chemotherapeutic agents into the arterial territory of malignant intracranial gliomas. Intra-arterial administration of chemotherapy has long been in focus as a treatment modality for treating brain tumors owing to the increased association of systemic drug toxicity and poor concentration within the tumor with intravenous administration. However, poor rationalization of drug injection protocol with the presence of high resting cerebral flow and an intact blood brain barrier has limited the translation of these advances into neuroendosurgical practice [1617].
CONCLUSION
The presence of collaterals and multiple feeders to brain tumors can create difficulty with regard to both forward pressure perfusion and application of therapeutic agents. Such tumoral vascular characteristics also increase the risk of inadvertent reflux into normal parenchymal arteries. In an illustrative case of a skull base meningioma, we demonstrate a double catheter technique with a combination of coiling and particle embolics. Coiling prevents reflux into normal collateral vascular territories, while promoting a concentrated forward perfusion pressure during particulate tumoral embosurgery.