Carotid Artery Stenting and Its Impact on Cognitive Function: A Prospective Observational Study
Article information
Abstract
Purpose
Carotid artery stenting (CAS) has evolved as a first-line therapeutic option for carotid revascularization in indicated patients for stroke prevention, but there is still a lack of data on its effect on cognitive function (CF), especially among Indian patients. To determine the effect of CAS on CF and to study the immediate and delayed complications of CAS in Indian patients.
Materials and Methods
This was a prospective, observational, single-center study. CF was assessed using Addenbrooke’s cognitive examination version III (ACE) before and 3 months after stenting. The demographic and clinical parameters were also assessed. A follow-up evaluation after 3 months was done to compare CF and to observe the occurrence of any complications.
Results
Out of 31 patients, 3 were lost to follow up. There were no immediate or delayed procedure-related complications. There was a statistically significant improvement in overall ACE score and memory before and after stenting. On subgroup analysis of those with and without strokes, there was a significant improvement in visuospatial function and mean ACE score. Those with left CAS had significant improvement in memory, visuospatial, language, and ACE scores than right CAS.
Conclusion
CAS was associated with significant improvement in CF in patients.
INTRODUCTION
With the aging of the population, the prevalence of cognitive impairment is increasing and has become a focus of research [1]. Without substantial success in the treatment of degenerative diseases of cognition, the present strategy concerns the targeted and optimal treatment of modifiable risk factors that lead to vascular cognitive impairment (VCI) [1]. Carotid atherosclerosis and carotid artery stenosis (CS) are leading causes of stroke in the elderly and thus may add to the burden of vascular dementia [2]. Even when asymptomatic, it may contribute to cognitive decline, possibly due to silent embolization and chronic hypoperfusion, which may make it an independent risk factor for VCI [1]. It follows that revascularization may halt or reverse cognitive dysfunction. However, revascularization procedures have also been implicated in a worsening of cognitive function because of increased microemboli, temporary flow interruption from clamping (carotid endarterectomy) or balloon dilatation (carotid artery stenting [CAS]), or hyper-perfusion (post-procedure) related injury [3,4]. This interaction between CS, revascularization, and cognitive dysfunction remains poorly understood, with the published data showing mixed results [1,3-9]. Hence, we conducted a study primarily to determine the effect of extracranial CAS on cognitive function and secondarily to study the immediate and delayed complications in Indian patients.
MATERIALS AND METHODS
This was a prospective observational study. Patients above 18 years of age who underwent stenting from June 2017 till November 2018 were included in the study. Patients with previously diagnosed dementia, less education, disabling strokes with a modified Rankin’s score 3 or more [10], aphasia, psychiatric illness, drug or alcohol abuse, stenosis due to a non-atherosclerotic cause or calcified lesions, or severe hepatic or renal dysfunction were excluded.
All patients previously diagnosed to have CS with either magnetic resonance angiogram or computed tomography or Doppler ultrasound underwent digital subtraction angiography to determine the severity of stenosis based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [11]. Measurements of severity, length of the stenosis, and plaque characteristics were performed to select a properly-sized balloon and stent. Stenting was performed by a single neurointerventionist under local anesthesia and through a femoral approach. A distal protection device was used to prevent microembolisms during the procedure in all cases. Patients with symptomatic stenosis more than 50% or asymptomatic stenosis more than 70% as per NASCET criteria underwent stenting.
Cognitive function of the enrolled patients was assessed using Addenbrooke’s cognitive examination version III (ACE) by a trained neuropsychologist before and 3 months after stenting [12]. Apart from cognitive function, demographic and clinical data of the study patients were also analyzed. A follow-up evaluation after 3 months was done to compare the cognitive function, occurrence of stroke or transient ischemic attack (TIA), renal failure, or other vascular events like acute myocardial infarction (MI) or death (due to any cause). Carotid Doppler was also done during their visit to diagnose restenosis and measure flow across the stent. Informed consent from each participant and approval of the institutional ethics committee was taken for the study (IRB no. NHH/AECCL-2017-185).
Statistical analysis
Categorical data was represented in the form of frequencies and proportions. Continuous data were represented as mean and standard deviation. The chi-square test was used as a test of significance for qualitative data. The independent t-test or Mann–Whitney U test was used as a test of significance to identify the mean difference between 2 quantitative variables and qualitative variables respectively. The paired t-test and Wilcoxon signed-rank test were used for paired data such as before and after for quantitative and qualitative data respectively. A P-value <0.05 was considered to indicate statistical significance.
RESULTS
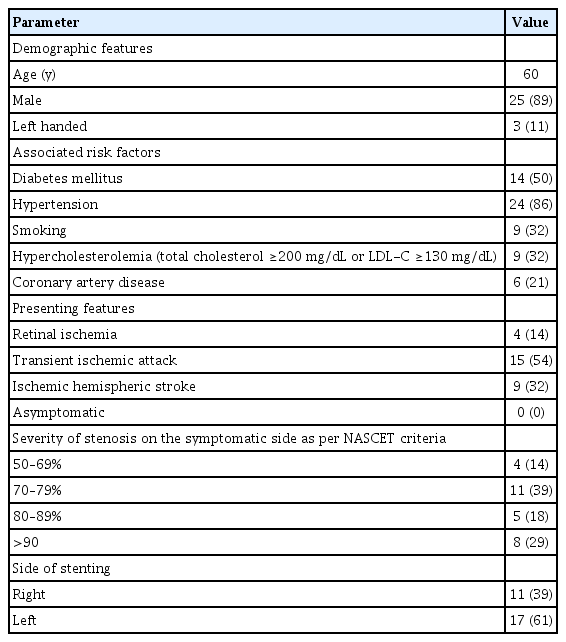
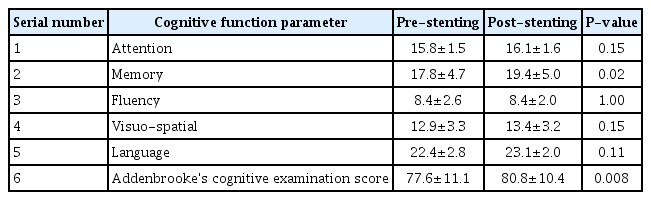
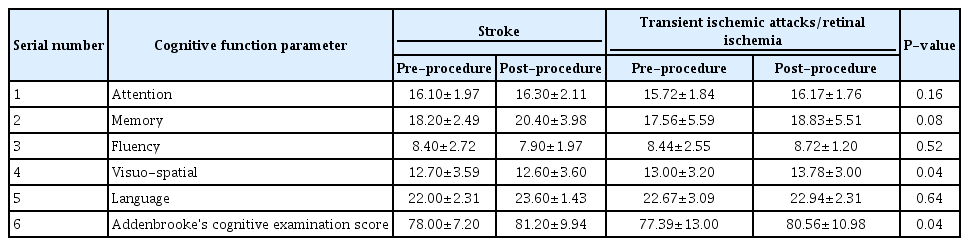
Thirty-one patients were enrolled during the study period out of which 3 were lost to follow up. The baseline clinical demographic features are as mentioned in Table 1. The pre- and post-stenting ACE scores and individual cognitive domain scores are compared in Table 2. Additionally, the cognitive function scores were compared between patients with stroke (established infarcts) and those with TIAs/retinal ischemic symptoms only, as shown in Table 3, and between left- and right-sided CAS procedures, as shown in Table 4.
There was no procedure-related complication or post-procedure event (stroke, TIA, myocardial infarction, or death). On follow up comparison between pre- and post-stenting cognition of the patients, there was a statistically significant improvement in mean ACE score (P<0.008) and memory (P<0.02). Between patients with and without stroke, there was a significant improvement in visuospatial function and mean ACE score (P<0.04, each). Among patients undergoing left-sided stenting, memory, visuospatial, language, and ACE scores were found to have significant improvement (with P-values less than 0.04, 0.02, 0.036, and 0.002, respectively) as compared to those who underwent right-sided CAS.
DISCUSSION
Determination of symptomatic or asymptomatic status of a CS does not take cognitive function of the patient into account. Though it is well known that stroke is a cause of dementia, carotid stenosis itself is yet not recognized as an independent risk factor for a cognitive decline [4,13]. The objective of our study was to determine this relationship between the effects of CAS on CF. We had used ACE score as it is widely applied in clinics as a battery for evaluation of cognitive function. In our study, we found that there was a significant improvement in the overall ACE score and memory after CAS. We also found significant improvement in memory, visuospatial, language, and overall ACE scores of patients who underwent left carotid stenting, which is expected as the left hemisphere is dominant in the majority of cases. A similar differential improvement between right- and left-sided stenting was observed in 1 study by Ishihara et al. [14]. The possible mechanisms may be related to restoration of flow to the chronically hypoperfused brain and prevention of further strokes by preventing the progression of local atherosclerotic lesions [3,4,8]. Some of the studies have also shown that stenting itself may be associated with deterioration of cognitive function [15], or associated with mixed results [4]. However, this is contrary to our study findings. One large prospective study from China also supports the findings of our study in which they found significant and sustained improvement in cognition after carotid stenting [9]. The absence of any major complication in our study may have contributed to better functional cognitive outcomes. The reported rate of complications (stroke, TIA and MI) during stenting in various studies has been 6% to 9% for symptomatic and 2% to 4% for asymptomatic patients [16-18]. The data on the rate of periprocedural complications amongst Indian patients during CAS is scarce. One study by Gupta et al. [19]. reported a periprocedural mortality rate of around 8.1% and minor stroke of about 4.1%. Another reason for the positive outcome could be the uniform procedure, namely, the use of a distal protection device and a single operator in all cases.
This was an observational study with a small sample size; hence, the results should be confirmed in a randomized controlled trial. Although the ACE is a validated tool for cognitive assessment, it may not reflect the overall cognitive function of patients. Confounding factors due to the practice effect may not be eliminated because of a lack of a control group. In addition, the study of regional blood flow and the effect of infarct location, as far as the eloquent nature of the cortex is concerned, must also be considered before cognitive decline is attributed to a carotid lesion. The risk factors of CS also contribute to vascular dementia [1,3]. Hence, the real contribution of CS in cognitive decline independent of the other risk factors must also be studied. Further large scale studies with standardized cognitive assessment are required for a better understanding of the complex nature of the interaction between carotid atherosclerosis and cognition.
CONCLUSION
Carotid atherosclerosis may contribute to the burden of cognitive impairment; stenting in indicated patients may help in prevention of cognitive decline.
Acknowledgements
We thank Ms Delitia Manuel for her valuable help with the statistical analysis of the study.
Notes
Fund
None.
Ethics Statement
Ethics approval was obtained from the NH Institute of Neurosciences Institutional Review Board (IRB no. NHH/AECCL-2017-185).
Conflicts of Interest
The authors have no conflicts to disclose.