Elevated Red Blood Cell Distribution Width May Be a Novel Independent Predictor of Poor Functional Outcome in Patients Treated with Mechanical Thrombectomy
Article information
Abstract
Purpose
Red blood cell distribution width (RDW) evaluates the variation (size heterogeneity) in red blood cells. Elevated RDW has been identified as a predictor of poor functional outcomes for acute ischemic stroke. The association between elevated RDW level and poor functional outcome in stroke patients undergoing mechanical thrombectomy has not been reported before. This study aims to investigate this relationship.
Materials and Methods
This was a multicenter retrospective study involving the prospectively and consecutively collected data of 205 adult stroke patients who underwent mechanical thrombectomy for anterior circulation large vessel occlusion (middle cerebral artery M1, anterior cerebral artery A1, tandem ICA-MCA, carotid T) between July 2017 and December 2019. RDW cut off levels were accepted as >16%. The effect of elevated RDW on poor functional outcome (modified Rankin scale 3–6) was investigated using bivariate and multivariate regression analysis.
Results
Elevated RDW was significantly associated with poor functional outcome in bivariate and multivariate analysis (odds ratio [OR] for RDW >16%, 2.078; 95% confidence interval [95% CI], 1.083–3.966; P=0.027 and OR for RDW >16%, 2.873; 95% CI, 1.342–6.151; P=0.007; respectively).
Conclusion
These findings suggest that elevated RDW may be an independent predictor of poor functional outcomes in ischemic stroke patients undergoing mechanical thrombectomy.
INTRODUCTION
Red blood cell distribution width (RDW) evaluates the variation (size heterogeneity) in red blood cell (RBC) volume, which is easily measured by a complete blood count test. RDW has been recognized as a robust prognostic marker for a variety of diseases, especially for acute vascular diseases [1,2]. The normal RDW range differs according to laboratory normalized values (11.5–14.5%; 11.5–16%) [3-6]. An elevated RDW level indicates greater and abnormal variation in RBC size in the peripheral blood, known as anisocytosis. Anisocytosis is generally caused by increased or ineffective production of RBCs and extreme fragmentation or destruction of RBCs resulting in thrombosis tendency [1,7].
Elevated RDW has been reported as an independent predictor of mortality in acute ischemic stroke (AIS) patients and is associated with a poor functional outcome in AIS [3,4,8-16]. We performed this study to investigate the prognostic value of RDW for AIS patients undergoing mechanical thrombectomy.
MATERIALS AND METHODS
Study design and patient selection
This was a multicenter retrospective study with the participation of 3 stroke centers involving prospectively and consecutively collected data of 245 adult patients with anterior circulation AIS between July 2017 and December 2019. After applying the exclusion criteria, 205 patients were included in the study.
All patients in the study population met standard acute stroke treatment criteria and received intravenous recombinant tissue plasminogen activator (IV rt-PA) in accordance with current stroke guidelines. Patients who did not qualify for IV rt-PA, mainly because of presenting outside the recommended time window, were considered for mechanical thrombectomy. The decision to proceed with thrombectomy as part of the treatment did not influence the administration of IV rt-PA and was based on the identification of a large vessel occlusion on computerized tomographic angiography in patients presenting within 6 hours of the estimated time of onset.
Exclusion criteria were as follows: <18 or >82 years, modified Rankin scale (mRS) >2, National Institutes of Health Stroke Scale (NIHSS) >25, malignancy, computerized tomography diagnosis of cerebral hemorrhage, intracerebral mass, or cerebrovascular damage secondary to trauma, presence of hemoglobinopathy (sickle cell anemia, thalassemia), anemias (hemoglobin <10 g/dL) [17], low ejection fraction (<35%), chronic kidney disease (creatinine >2 mg/dL), and posterior circulation large vessel occlusion and evidence of bone marrow insufficiency. RDW levels were measured before mechanical thrombectomy. Patients were grouped according to RDW level (cutoff value for RDW was defined as 16% according to the normal range of our RDW kit). Accordingly, patients were divided into 2 groups: RDW ≤16 and RDW >16. The groups were analyzed for poor functional outcome.
Written consent was obtained from the patients or their next of kin (for aphasic or unconscious patients) for participation in the study and the study was approved by the ethics committee at Samsun Training and Research Hospital.
Data collection
All clinical and demographic characteristics (sex, age, presence of hypertension, diabetes mellitus, current smoking, hypercholesterolemia, and coronary artery disease), laboratory results, and radiological findings of the patients were recorded. Initial stroke severity was evaluated based on NIHSS recorded at the time of admission. The primary outcome was poor functional outcome at 90 days, as reflected by an mRS score of 3–6. Successful revascularization was defined as post-treatment modified thrombolysis in cerebral ischemia (mTICI) score 2b–3.
Laboratory parameters
RDW normal values were defined between 12% and 16% according to our laboratory. RDW cut off levels were accepted as >16%. A complete blood count test, including RDW measurement, was performed with peripheral venous blood samples on admission.
Statistical analyses
All variables were tested for distribution normality using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were expressed as means±standard deviation. Categorical variables were expressed as numbers (percentages). Categorical data were compared using the chi-square test. Logistic regression was used to evaluate independent effects of RDW on the outcome. Multivariate regression analysis was performed to evaluate possible correlations of RDW with functional outcome (mRS at 3 months), stroke severity (NIHSS), age, and location of occlusion (MCA, ICA, basilar). The odds ratio (OR) and its 95% confidence interval (95% CI) were calculated for the variables found to be significantly associated with the endpoint in the multivariate model test. All analyses were performed using SPSS 11.0 (SPSS Inc., Chicago, IL, USA), and P<0.05 was defined as statistically significant.
RESULTS
After the exclusion criteria was applied, 205 patients undergoing mechanical thrombectomy were included in the study. The mean age was 63.7±11.5 years and 95 (46.3%) were females. The mean RDW level was 13.7±2.2. Successful recanalization (mTICI 2b-3) occurred in 162 cases (79%). The time between symptom onset and groin puncture was 271.2±79.4 minutes (128–445). The mean NIHSS score upon admission was 14.2±6.4 (range 6–25, median 14). A total number of 84 (40.9%) patients had a poor functional outcome after 3 months from acute ischemic stroke. We identified tandem ICA-MCA occlusion in 53 (21.6%) patients, carotid T or L occlusion in 65 (26.5%) patients, M1 occlusion in 123 (50.2%) patients, and anterior cerebral artery A1 occlusion in 4 patients (1.6%). Table 1 summarizes the main demographic and clinical data of the patients.
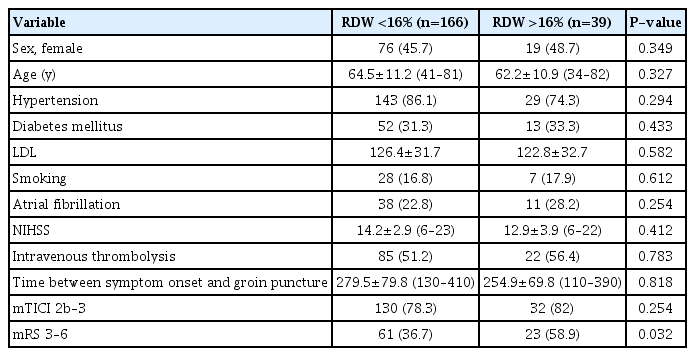
Baseline characteristics of acute stroke patients undergoing mechanical thrombectomy according to RDW
In multiple logistic regression analysis, RDW value higher than 16% and mTICI score 1–2a were significantly related to poor functional outcome (Table 2).
Elevated RDW was found to be significantly associated with poor functional outcome in bivariate and multivariate analysis (OR for RDW >16%, 2.078; 95% CI, 1.083–3.966; P=0.027 and OR for RDW >16%, 2.873; 95% CI, 1.342–6.151; P=0.007, respectively) (Table 3).
DISCUSSION
To the best of our knowledge, this is the first study to investigate the association between elevated RDW and poor functional outcome in patients with acute ischemic stroke undergoing mechanical thrombectomy. We found that elevated RDW could significantly predict poor functional outcome in patients with acute ischemic stroke who underwent mechanical thrombectomy. However, the underlying mechanism to explain the prognostic value of RDW for AIS remains unknown.
Tonelli et al. [16] first described the potential association between RDW and cardiovascular disease (including stroke) in 2008. This was an analysis of data from the Cholesterol and Recurrent Events study with 4,111 participants followed for 60 months. This study showed that a 1% increase in RDW was associated with a 20% increase in stroke risk. Two other studies confirmed increased RDW was associated with increased stroke risk [13,15]. The prognostic value of RDW was demonstrated by a small population study. Kara et al. [14] demonstrated significantly higher RDW values in stroke patients compared to the control population (14.7 vs. 13.6%; P=0.001).
Among stroke patients, severe stroke patients that had higher NIHSS scores had significantly higher RDW values when compared with mild stroke patients (15.9 vs. 14.2%; P<0.05). Another study published by Ani and Ovbiagele concerning the prognostic value of RDW in ischemic stroke patients showed that baseline RDW value was higher in patients that died (13.9 vs. 13.4%; P<0.001) [4]. Our results are consistent with previous studies. They demonstrated that RDW >16% is significantly associated with poor functional outcome (P=0.032).
Despite previous studies demonstrating an association between RDW and poor functional outcome in ischemic stroke, it is still unknown whether RDW is a triggering factor or a result of a complex pathophysiologic mechanism for poor functional outcome in stroke. Inflammation and oxidative stress may have important roles [18]. Inflammatory cytokines, such as interleukin-1, tumor necrosis factor-α, and interferon-γ, which are known to be released in chronic inflammatory states, could affect bone marrow red blood cell production, maturation, and could subsequently lead to anisocytosis Inflammation can reduce the survival rate of red blood cells and inhibit red blood cell production, which can lead to red cell damage [19]. Oxidative stress, which is a result of an imbalance between in vivo oxidation and antioxidation, causes damage to protein and lipid structures, which could also lead to red blood cell damage along with increased red blood cell fragility, resulting in elevation of RDW [20]. Therefore, disturbance of erythropoiesis may worsen the ischemic symptoms. Erythrocytes with high RDW values may circulate less in the microcirculation, resulting in decreased tissue oxygenation, which may facilitate the rate of penumbral loss in acute stroke resulting in poor functional outcomes. Another proposed mechanism is inhibition of nitric oxide activity in the presence of anisocytosis, which results in reduced dilatation of flow-dependent arteries [21]. It would be valuable to have data on peripheral oxygenation, but unfortunately, these data were not recorded for all patients.
RDW is a simple, easily applicable, laboratory test assessed rapidly by automated cell counters. A possible association between RDW and functional outcome would allow further means to predict prognosis in acute stroke. Further studies with a prospective design and a larger sample size are needed to investigate the prognostic value of RDW for AIS patients undergoing mechanical thrombectomy.
In our study, RDW was measured only once. As RDW may change in some conditions, such as acute blood loss, transfusion, infection, inflammation, and renal dysfunction, more than 1 measurement at different times might produce more reliable results. A second limitation is this study only included patients anterior circulation stroke.
CONCLUSION
Although it is uncertain whether increased RDW values are a consequence of or a contributing factor in acute stroke pathogenesis, our study is the first to show the predictive role of RDW for poor functional outcome in acute ischemic stroke patients undergoing mechanical thrombectomy. More studies are required to evaluate and validate this finding.
Notes
Fund
None.
Ethics Statement
Written consent was obtained from the patients or their next of kin (for aphasic or unconscious patients) for participation in the study and the study was approved by the ethics committee at Samsun Training and Research Hospital.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contribution
Conception, study design, interpretation of the data, and manuscript composition: CKA, AOO, and EG. Data collection, instructions for data analysis, manuscript composition: OA and ZU.


