Obesity and Stroke: Does the Paradox Apply for Stroke?
Article information
Abstract
Historically, obesity has been identified as one of the most important risk factors for developing cardiovascular diseases including stroke; however, a theory called “The Obesity Paradox” has been recently considered. The paradoxical theory is that obese or overweight patients (according to body mass index score) can have better outcomes compared to leaner or malnourished patients. The paradox was initially discovered in patients with heart failure. The purpose of this manuscript was to investigate whether this paradox also applies to stroke patients, according to information available in the current literature.
INTRODUCTION
Worldwide, stroke is the second leading cause of death and obesity is the second leading preventable cause of death. Obesity is a risk factor for multiple cardiovascular diseases, such as coronary disease, coronary death, congestive heart failure, and stroke [1,2]. In China, stroke is the main cause of major disability and mortality [3]. During the last 40 years, the world prevalence of obesity has nearly tripled. By 2016, the World Health Organization reported 38% of men and 40% of female patients (1.9 billion) were overweight, and 11% and 14% (600 million) were obese, respectively [4]. In the United States, annual medical spending has considerably increased due to excess weight and was estimated to reach nearly USD 80 billion in 1998 and USD 147 billion in 2008 [5]. Moreover, between 2000 and 2005, the medical cost for obesity-related illness was estimated to be more than 200 billion, equivalent to more than a fifth of the national health expenditure [6]. Therefore, the need to elucidate the relationship between obesity and most diseases is progressively increasing. There is very little evidence addressing cerebrovascular diseases and obesity, other than the relative risk of occurrence of the disease. Studies are more focused on stroke prevention than on prognosis in obese patients. Pathophysiological mechanisms underlying the effect of obesity on stroke remain unclear and controversial. There is a recent theory called “The Obesity Paradox” that states that patients with an elevated body mass index (BMI) might present better clinical outcomes after a heart failure, although the information regarding its relation with stroke is scarce. The objective of this manuscript is to provide an overview on whether there is a relationship between the obesity paradox and stroke, along with its neurointerventional implications, by reviewing the current literature.
THE CURRENT GLOBAL PROBLEM OF STROKE AND OBESITY
Stroke is an alteration in cerebral perfusion due to multiple causes and is the second leading cause of death and disability worldwide. Its frequency has increased recently in low-and middle-income countries and decreased in high-income countries [7-12], and its incidence doubles in individuals older than 55 years of age [13]. The diagnosis is made based on neurological signs and symptoms that provide evidence on the affected vessel and corresponding brain region [10,14-16]. Usually, the symptoms include unilateral extremity weakness, numbness, visual alterations (e.g., blurred vision, diplopia, or binocular blindness), alteration in speech, vertigo, hemiballismus, and alien hand syndrome [14]. Depending on injury magnitude, tissue compromise, and opportune medical management, patients can present with multiple lethal complications that may lead to unfavorable outcomes [17,18]. There are multiple risk factors for stroke development like arterial hypertension, hypercholesterolemia, diabetes mellitus, cardiopathy, tobacco or excessive alcohol consumption, environmental air pollution, high-risk diet, and obesity [12,14,27,19-26].
Obesity is the second leading cause of preventable death (after tobacco) worldwide and is considered a public health problem with a doubled prevalence since the 1980s, due to the globalization of its risk factors and an exponential increase in its consequences [11,23,24,27-33]. Associated with the high prevalence are the changes in diet, sugar intake, chemicals added to products in food treatment, bigger portion size, low/inadequate physical activity, bad eating habits, more processed food, and foods with higher caloric content [8,28,33]. Altogether, they have contributed to the weight gain in the population that lead to the fact that obesity is currently considered a pandemic [8,28,34]. Obesity is diagnosed as a BMI >30 kg/m2 (calculated by the patient’s weight in kilograms divided by the squared height in meters) [20,22,29,30,35,36]. However, it is important to consider the world regions and countries, as the measurement can change somewhat [8,12,28,37].
BMI AND OBESITY
The current problem with the BMI measure is that it does not give a precise idea about body composition, which affects health risks of excess weight such as the proportion of bodyweight and the distribution of fat [23,32,38-40]. BMI has been criticized because it does not differentiate fatness, obesity, and adiposity measurement [11,29,32,39,40]. Other methods, including waist circumference and central and peripheral fat mass, have also been proposed for the diagnosis; still, BMI continues to be used for the classification of obesity [23,32,41]. Individuals are classified as overweight if their BMI is between 25 kg/m2 and 30 kg/m2, obese if it is between 30 and 40 kg/m2, and morbidly obese if it is greater than 40 kg/m2 [40,42,43]. BMI is not used for children and adolescents from 2 to 18 years; instead, it is recommended that a percentile scale based on the child’s sex and age be used. In this population, overweight is defined as a BMI in the 85th to 94th percentile, and obesity is a BMI at or above the 95th percentile [42,43]. In the INTERHEART study, BMI was related to the risk of myocardial infarction, but this relation was weaker than that of abdominal obesity (waist-to-hip ratio or WHR), with BMI becoming non-significant with the inclusion of WHR in the multivariate model [44]. In addition, a study in 32 countries, whose objective was to determine the risk factors of stroke (INTERSTROKE study), showed a weaker association with the WHR [45]. However, it is known that each unit of BMI is independently associated with a 6% increase in the relative risk of stroke [46]. Even though there are other measurements than BMI, they do not show an obesity paradox, but a linearly increased mortality association is evidenced [40].
THE OBESITY PARADOX
As we previously mentioned, obesity predisposes an increased risk of suffering cardiovascular and systemic diseases, such as stroke and other diseases [29,33,34,47-49]. It is associated with premature mortality; and, in multiple studies, a higher risk of cardiovascular disease incidence has been demonstrated in underweight adults or just overweight (but not obese) patients. As a result, there is no clear evidence of a higher prevalence or incidence of stroke in those with an obesity diagnosis [37,47]. The “Obesity paradox theory” says that patients with high BMI could have a better prognosis than leaner patients (lean paradox) regarding clinical outcomes in those with cardiovascular diseases [22,25,30,40,50]. This means that even when obesity leads to higher complications and risk of suffering from multiple diseases and complications, high adiposity can present a protective role against infections and, regarding stroke, better mortality and outcomes [11,23,25,34,47].
Some studies show that for every 5-unit increase in BMI above 25 kg/m2, overall mortality increases by 29%, vascular mortality by 41%, and diabetes-related mortality by 210% [21,51]. In 2001, Horwich et al. [26] coined the term “Obesity Paradox” to describe these findings correlating an increase in BMI with better prognosis and clinical evolution of patients with heart failure [22,23,30,52]. Two years later, Lavie et al. [53] found that every 1% increment in body fat percentage will correspond with a 13% reduction in cardiovascular events [25,30]. The “fat but fit” phenomenon was proposed to explain those obese patients without metabolic derangements whose outcomes were unfavorable [54].
The obesity paradox can be explained by the protective effect of a major endocrine organ known as adipose tissue [22,47], which secretes soluble TNF-alpha-receptors and neutralizes the impact of tumor necrosis factor alpha (TNF alpha) in the human biological system and inflammatory responses [23,26,30,47]. Overweight and obese patients have higher lipoproteins and lipids serum levels, and these play an important role in detoxifying and binding lipopolysaccharides and are related to inflammatory cytokines cascade blocking. Further, these molecules impede the inflammatory state after a stroke episode [30,32,47]. Knowing that inflammation and immune mechanisms are risk factors and outcome predictors in stroke, is important to consider that obesity has an inflammatory component and is known as a low-grade chronic inflammatory condition due to the C-reactive protein, TNF alpha, soluble intracellular adhesion molecule-1 and interleukins (the proinflammatory markers are low in the obese compared to infectious situations but remain elevated in longer periods) [12,20,30-32].
ADIPOSITY AND LIPOTOXICITY IN STROKE
Adipocytes produce adipokines, and these hormones play a protective role in the myocardium, but pro-inflammatory cytokines like interleukin-1b and 18, TNF-alpha and some others, lead to diastolic dysfunction [21-23,26,29,30,32]. Lipotoxicity (a term originally used to describe the destructive effects of excess fat accumulation on glucose metabolism) causes functional impairments in several metabolic pathways, both in adipose tissue and peripheral organs, like the liver, heart, pancreas, and muscle. They also play an important role in insulin resistance and pancreatic beta-cell dysfunction [26].
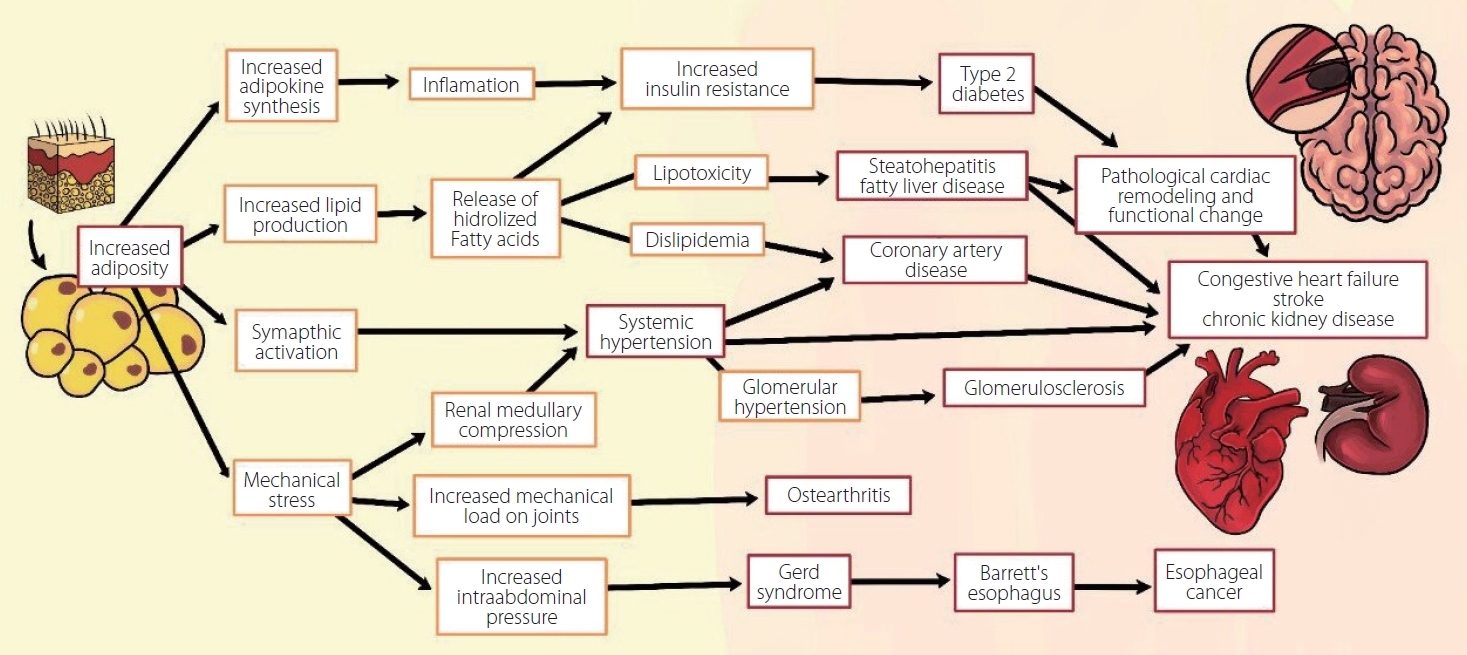
The lipid peroxidation and hydrolysis of free fatty acids (FFA) in the liver and vascular endothelium, along with high levels in patients with obesity, promote FFA transport to peripheral tissues and increments of total body fat. This generates a higher concentration of adipokines and various hormones that activate the pathogenesis base pro-inflammatory pathway of atherosclerosis and its chronic complications (stroke, myocardial infarction, etc.). These hormones block second messengers favoring insulin resistance (Fig. 1) [21,22,26,27,52,55,56]. In addition to being found in adipose tissue, lipids are also found in liposomes, which are small cytoplasmic organelles in proximity to the mitochondria in many types of cells. Furthermore, accumulation of excess lipid intermediates (e.g., ceramides) in some adipose tissues can lead to lipotoxicity with cellular dysfunction and apoptosis in all types of cells (including neurons and neuro-vasculature cells), and this is the base for the development of neurodegenerative and cerebrovascular diseases [26]. The excess production of fatty acids increases adipose tissue and pro-inflammatory products resulting from lipid peroxidation, together with atherosclerosis, which leads to an increase in vascular resistance and a state of chronic sympathetic hyperactivity. This influences the pathogenesis of many of chronic diseases [47,52,55]. Fig. 2 shows various pathways of chronic disease development that involve adiposity and lipotoxicity [57].
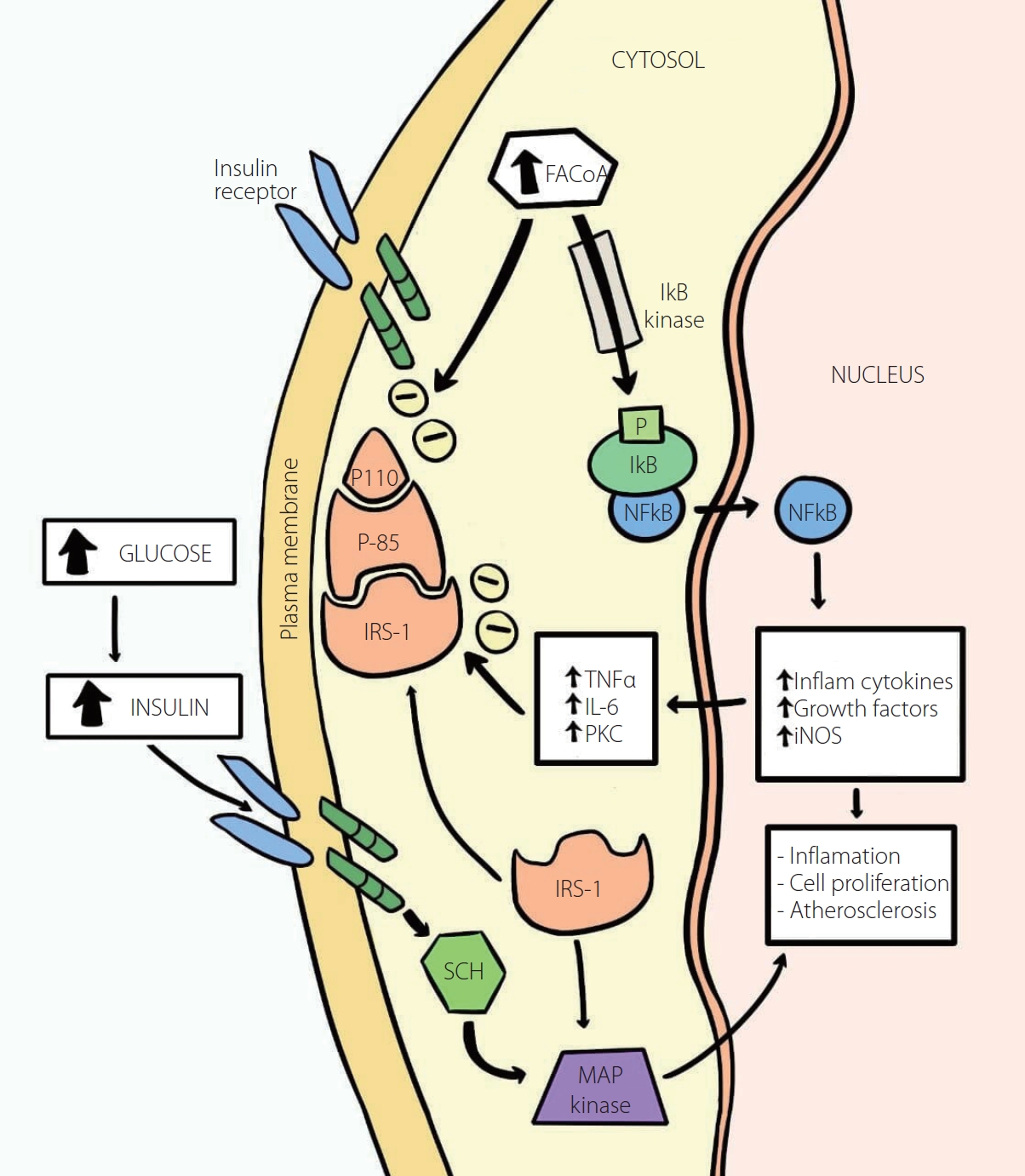
Increased activity of IkB/NFkB as the basis of the chronic inflammation and insulin resistance in type 2 diabetes. Intracellular fatty acyl-CoA levels found in insulin resistance and lipotoxicity, in correlation to the subsequent activation of the enzyme IkB kinase (via inflammatory factors such as fatty acyl-CoAs), will phosphorylate IkB, causing the release of NFkB (upon the polyubiquitination and degradation of IkB), which enters the nucleus where it is responsible for inflammation, cell proliferation and atherogenesis by the stimulation of inflammatory cytokines, growth factors, and iNOS, which in turn will stimulate TNFα, IL-6, and PKC, which will impede insulin signaling by serine phosphorylation of IRS-1 ultimately, causing insulin resistance. Therefore, the increased activity of IkB/NFkB will not only instigate inflammation but also further aid and boost atherogenesis. The MAP kinase pathway is responsible for the insulin-stimulated glucose impaired metabolism and for this reason, related to cardiovascular diseases. FACoA, Fatty acyl-CoA; IkB, Inhibitor kβ; IL1 and IL6, Interleukins 1 and 6; IRS, Insulin Receptor Substitute 1; MAP, Mitogen-Activated Protein; NFkB, Nuclear factor kβ; NO, Nitric Oxide; NOS, Nitric Oxide Synthase; PI3K, Phosphoinositide 3-kinase; PKC, Protein Kinase C; SCH, Src homology 2 domain; TNFα, Tumor Necrosis Factor alpha; Inflam, Inflammatory. Adapted and modified from the article of Yazici and Sezer (Adv Exp Med Biol 2017;960:277-304) [56].
CURRENT EVIDENCE FOR THE OBESITY PARADOX AND STROKE RELATIONSHIP
As mentioned before, obesity predisposes a risk for developing vascular diseases like stroke [7,36,47,58]. Further, it has been established that for a BMI greater than 20, an increase by every unit adds to the ischemic stroke risk by 5% [7,47]. The relation between stroke risk increase in obese patients is related to the amount of adipose tissue and a repository of inflammatory cells that lead to subsequent atherosclerosis promoted by hyperglycemia and insulin resistance [7,21,31]. In some studies, it has been shown that obese patients could present with mild ischemic strokes due to small vessel occlusion and have better functional outcomes, which means those patients have a lower mortality risk [9,58]. Table 1 presents additional studies regarding the beneficial or favorable relationship of the obesity paradox with stroke [59-65].

Additional studies regarding the beneficial or favorable relationship of the obesity paradox for stroke in humans
However, some studies indicate that for obesity class III (40 to 49.9 kg/m2) the paradox is no longer applicable due to the U or J shaped effect [30]. Further, there is a clear association between BMI-related mortality and this effect [40]. In this case, these effects show that depending on the “nutritional status” at the extreme points, the paradox (that is the reason why it is a U or J shaped effect) is not useful, as each extreme point (morbid obesity or malnourished) has a higher risk of incidence, unfavorable outcomes, and mortality. The intermediate point or low curve will be obesity. By this, it could be presumed that being obese does not necessarily lead to a higher stroke incidence [20,23,34,37,39,40,47,66]. Some studies have reported a “protective effect” on stroke outcomes in obese patients. Higher BMI has been related to lower long-term mortality and higher improved outcomes after a stroke episode when compared to patients with a normal BMI [20].
More recently, in patients suffering from stroke and intracerebral hemorrhage, the evidence is less clear regarding BMI and its association with complications and outcomes in aneurysmal subarachnoid hemorrhage (SAH). In the TEMPiS (Telemedical Project for Integrative Stroke Care) trial study where clinical outcomes (mortality and good neurological prognosis) were evaluated and grouped according to BMI, mortality was significantly lower in obese patients (all BMI 30 kg/m2) than in patients with normal weight (hazard ratio: 0.70; 95% confidence interval: 0.50–0.98) [27,55]. In conclusion, The trial study showed that overweight patients with acute stroke or TIA (Transient Ischemic Attack) compared to lean or malnourished patients presents a better outcome and less dependency [27].
A systematic review by Oesch et al. [47] evaluated 25 studies in which 299,700 patients participated, and found 10 of 12 studies (162,921 patients) reported significantly fewer mortality rates in stroke patients with higher BMI values. In total, 7 of 9 studies (92,718 patients) showed a favorable effect of excess body weight on non-fatal outcomes (good clinical outcome, recurrence of vascular events). In 6 studies (85,042 patients), contradictory results after intravenous thrombolysis were seen; however, several methodological limitations were observed in a major part of the studies (observational study design, inaccuracy of BMI in reflecting obesity, lacking body weight measurement, selection bias, survival bias) [22,47].
Some other studies also reported an association between the paradox and stroke. A study by Rodríguez-Castro et al. [31] measured obese and non-obese patients with stroke and showed that obese patients present better neurological impairment recovery but do not present worse clinical evolution after stroke compared to non-obese patients, Kim et al. [67] showed an obesity paradox evidenced after 90 days post-stroke where obese patients showed less mortality than leaner patients.
Very few studies regarding obese patients and the paradox relation with stroke reach an adequate level of evidence. For example, Persaud et al. [65] in 2019 showed that obese patients with intracranial hemorrhage had a better survival rate during the in-hospital stay, and they credited this result to statins intake and its pleiotropic effect with respective anti-inflammatory and anti-thrombotic properties that might reduce the infarcted area and increase survival rates. Regarding the relation of BMI to outcomes after a surgical procedure, the results might be a little controversial, and this may be because of the difference in the treatment and its frequency [68].
NEUROINTERVENTIONAL IMPLICATIONS
While endovascular implications and the obesity paradox are well-depicted in cardiovascular disease [69], in cerebrovascular diseases the evidence is scarce. Obesity Class I (BMI from 30 to 34.9) has been shown to reduce the risk of stroke and mortality following a carotid endarterectomy procedure due to occlusion and stenosis of the carotid artery. Even though these results could not be corroborated with the lean paradox because of not reaching significance in the analysis, only Class I obesity had better outcomes, but the increase in BMI was associated with a higher risk of cardiac arrest [70]. To reinforce this theory, the MR CLEAN trial underwent a post hoc analysis (n=366) and concluded that higher BMI improved functional outcomes, lowered mortality, and reduced recurrence of stroke progression after endovascular treatment of acute stroke due to large vessel occlusion [71]. Overweight and obese patients were also associated with a better 3-month recovery after stroke [72]. However, these results are not always reproducible as seen in Bouslama et al. [73] (n=926), which could not find an association between BMI and outcomes after mechanical thrombectomy due to large vessel occlusive acute ischemic stroke. Also, Chen et al. [74] (n=248) reported that metabolic syndrome (which measures obesity according to waist circumference) was associated with increased risk of unfavorable functional outcome at 90 days in ischemic stroke treated with endovascular treatment, but was not associated with mortality. However, this study had a small sample size and metabolic derangement ascribed to metabolic syndrome had a stronger influence on outcomes more than pure obesity. Therefore, to correctly apply the obesity paradox, the “fat but fit” phenomenon should be also taken into account. Branscheidt et al. [75] showed no association of obesity and outcomes in ischemic stroke treated by thrombolysis. However, the small sample size has to be highlighted as well. The lean paradox has to be included in the obesity paradox concept as well. In other series, Rinaldo et al. [68] reported that depending on the procedure (clipping or coiling) when treating an acute SAH, the outcome could change according to BMI values. An elevated BMI was associated with an increased odds of an unfavorable outcomes when the patient was treated with a clipping procedure but with a decreased odds when treated with the coiling procedure. This study also established that the increased and decreased odds were related to hypodensities detection (infarction zones) after the treatment, which could be associated with a mechanism mediated by the patient BMI that could affect the outcome prognosis, as the coiling procedure in patients with high BMI was associated with a reduced incidence of hypodensities [68]. The study of Dasenbrock et al. [76] concluded that adiposity might not interfere with the patient’s outcome after a SAH. Hughes et al. [55] performed an analysis of patients with an elevated BMI that were treated with clipping or coiling procedures and found that there was no difference in the functional outcomes after the procedure. The retrospective analysis of Platz et al. [77] did not found any correlation between BMI and the outcome after a SAH. Also, according to the results of the study made by Schultheiss, obesity might not be important regarding the outcome variables in patients who needed cranial surgery [78]. These statements were also evidenced by some other studies [79,80]. Even the use of statins to modify the metabolism of cholesterol has not shown any benefit unlike patients receiving statins after endovascular treatment for atherosclerotic lesions in coronary, peripheral, and cerebrovascular circulations. The patients receiving statins after a flow diverter did not benefit from the same [81,82]. Despite the evidence, further studies are needed to clearly outline recommendations for performing endovascular treatment in obese patients.
CONCLUSIONS
Stroke incidence is increasing and represents a high socio-economic impact on healthcare costs [83]. This implies a loss of life quality and daily life activities due to its severe sequelae. The management of stroke in obese patients is a challenge. We must carry out the individualization of established treatments, even while the Obesity Paradox makes it look like a good protective factor. Still, the management of risk factors (for stroke and obesity) is related to a higher reduction in incidence and recurrence for stroke [7]. Obesity is widely accepted and known as a cardio-cerebrovascular risk factor, so it is recommended that adequate body weight control (BMI <25 kg/m2) is maintained as primary prevention, as well as a secondary prevention [9]. Due to the U-shaped effect, we can assume that the Obesity Paradox is only applicable to Class I and II, but it is important to bear in mind the importance of treatment and prevention of obesity, especially to avoid getting into class III or greater [30,84]. Distinguishing between pure obesity and a metabolic derangement is essential in interpreting the results since this is clearly proven to produce poor outcomes, as seen with the metabolic syndrome. Furthermore, the integration of concepts should be properly applied during the design of a study. The Obesity Paradox should always coincide with the fat but fit phenomenon and the lean paradox. Regarding the pure Obesity Paradox, there is a lot of controversial information on this topic; however, studies that support the theory of the Obesity Paradox present many methodological and quality limitations. For the moment, there is not a clear relationship between the Obesity Paradox in stroke patients, and quality studies are needed to answer this question, especially if a relationship between stroke and obesity is investigated. As a matter of fact, higher sample studies regarding neurosurgical intervention and obesity are needed to establish stronger evidence, regardless of whether there is or is not a correlation between the paradox and stroke.
Notes
Fund
None.
Ethics Statement
This study waived approval of the institutional ethics committee.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contribution
Concept and design: EG, AA, and GAQ. Writing the article: EG, GAQ, and CL. Critical revision of the article: LRM, WAF, EG, and CL. Final approval of the article: EG and AA.