Endovascular Treatment of Arterial Steno-Occlusive Lesions in Symptomatic Moyamoya Disease
Article information
Abstract
The efficacy and safety of endovascular treatment (EVT) for moyamoya disease (MMD) have rarely been investigated. The objective of this study was to summarize the clinical outcomes of EVT for MMD and determine the potential role of EVT in treating symptomatic steno-occlusive lesions in MMD. Reports from January 2000 to December 2021 describing EVT in MMD were collected through a literature search. The search terms included “moyamoya”, “stent”, “angioplasty”, and “endovascular”. Data regarding baseline demographics, previous medical history, treated vessel, periprocedural complications, and angiographical recurrence were retrieved. This review included 10 studies with details of 19 patients undergoing a total of 31 EVT procedures. Twenty-one EVTs were performed as initial treatments for MMD, and 10 were performed as additional treatments for angiographical recurrence. The mean follow-up period of the initial EVTs was 9.0±11.9 months, with angiographical recurrence in 11 (68.8%) cases. The mean follow-up period of additional EVTs was 4.3±3.9 months, and seven (70.0%) EVTs showed restenosis of the re-treated vessel. Across all initial and additional EVTs, there were no differences in characteristics between the recurrence and non-recurrence groups. Overall, two periprocedural complications (9.5%) occurred, one vessel rupture and one massive intracerebral hemorrhage with subarachnoid hemorrhage. EVT plays a limited role in the management of symptomatic intracranial arterial steno-occlusive lesions of MMD. Recent advances in understanding the pathomechanism of MMD may urge neuro-interventionists to find a new endovascular approach with better balloon angioplasty or stenting mechanisms.
INTRODUCTION
Moyamoya disease (MMD) is a progressive steno-occlusive disease whose pathogenesis has not been well studied. MMD is known to cause the occlusion of terminal internal carotid arteries (ICAs), leading to recurrent strokes. The prevalence and incidence of MMD have recently gradually increased, especially in East Asia [1]. Although several trials did not show the efficacy of revascularization procedures in symptomatic MMD, revascularization or flow-augmentation surgery have been the standards of treatment for preventing recurrent stroke to date [2-4].
Although histopathological differences between typical atherosclerotic stenosis of the intracranial arteries and the steno-occlusive lesions of MMD are known [3], endovascular revascularization of lesions could be considered, especially when steno-occlusive lesions show focal or short segment involvement. Considering the current lack of effective medical management and the higher surgical risk of flow-augmentation, endovascular treatment (EVT) could be considered a viable option. However, EVT for MMD is not frequently performed, with only a few case reports published [5-14]. Therefore, the efficacy and safety of EVT for MMD remain unclear. The objective of this study was to summarize the clinical outcomes of EVT for MMD and determine the potential role of EVT in treating symptomatic steno-occlusive lesions in MMD. We included data such as treatment modality, type of stent, periprocedural complications and outcomes, and long-term angiographical and clinical outcomes to assess the potential of EVT for the treatment of symptomatic steno-occlusive lesions in MMD.
MATERIALS AND METHODS
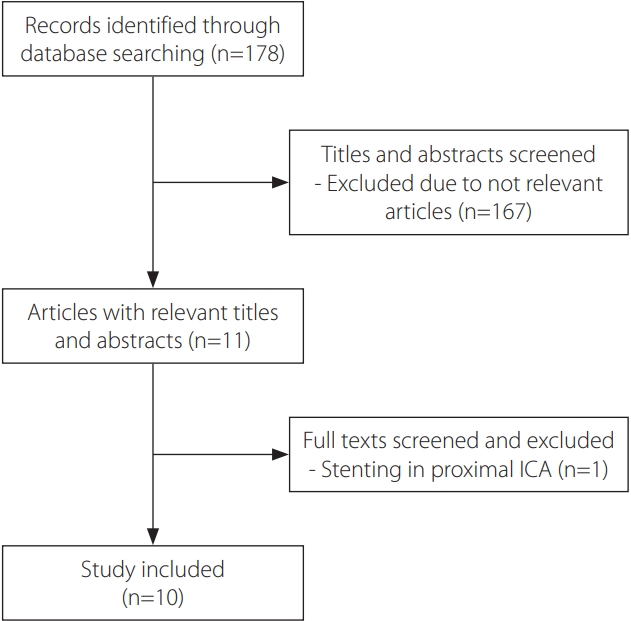
For a review of cases of MMD managed by EVT, we performed comprehensive searches of the PubMed and Embase databases. The search terms used were “moyamoya”, “stent”, “angioplasty”, and “endovascular”. Eleven studies from January 2000 to March 2022 were extracted, and one study associated with extracranial carotid artery stenting in MMD was later excluded. Therefore, a total of 10 reports were included.
We extracted the patients’ data, including age, sex, previous medical history, moyamoya type, the vessel with severe stenosis, treated vessel, treatment method (balloon angioplasty or stent deployment), occurrence of periprocedural complications, follow-up period after EVT, and occurrence of in-stent restenosis. For patients who underwent EVT more than once, we analyzed each procedure separately.
Moreover, we compared clinical characteristics according to angiographical recurrence. The significance of the intergroup differences was assessed using chi-square test or Fisher’s exact test, as appropriate. A P-value <0.05 was considered to be statistically significant.
RESULTS
A total of 10 reports, consisting of two case series and eight case reports, were collected in this analysis (Fig. 1). The baseline characteristics of reviewed patients who underwent balloon angioplasty or stent deployment for MMD have been summarized in Table 1, along with the assigned patient numbers (denoted by #). Overall, 19 patients were included, with 21 EVTs performed during the initial treatment (nine procedures with balloon angioplasty only, four with stent only, and eight with both treatments). All EVTs were performed on the affected vessels. Among the patients, two were children with medical co-morbidities (essential thrombocytosis in patient #1 and multiple congenital disorders in patient #4), and six of the 17 non-pediatric patients had vascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, or a history of smoking tobacco.

Baseline characteristics of reviewed patients who underwent balloon angioplasty and/or stent deployment
Among the 21 EVTs performed as initial treatments, the treated vessel was the ICA in 11 procedures and the middle cerebral artery (MCA) in 10 (Table 2). The Wingspan stent system (Stryker Neurovascular, Fremont, CA, USA) was the most used stent (10 of 12 cases). Coronary balloon-expandable stents were used in two patients, a coronary stent (AVE INX 3; Medtronic, Santa Rosa, CA, USA) in one patient, and a drug-eluting stent (DES) (Xience; Abbott, Chicago, IL, USA) in one patient. Overall, two periprocedural complications occurred. One patient who presented with a near-occlusion of the MCA experienced a catastrophic vessel rupture, and another with severe ICA stenosis experienced intracerebral and subarachnoid hemorrhages. The mean follow-up period of the 16 procedures with available follow-up data was 9.0±11.9 months. During the follow-up period, restenosis in the treated vessels occurred in 11 of 16 cases (68.8%).

Procedural factors and prognosis of reviewed patients who underwent first balloon angioplasty and/or stent deployment
For the treatment of angiographical recurrence, additional EVTs were performed in 10 cases (Table 3). Most procedures involved only balloon angioplasty (80.0%), one case of a stent only (10.0%), and one with both balloon angioplasty and stenting (10.0%). Although there were no periprocedural complications after retreatment, seven of the ten (70.0%) showed restenosis after a mean follow-up period of 4.3±3.9 months.

Procedural factors and prognosis of reviewed patients who underwent balloon angioplasty and/or stent deployment for instent restenosis after initial endovascular treatment
Considering all the cases of initial and additional EVT procedures taken together, we compared the clinical variables according to the angiographical recurrence (Table 4). There were no differences between cases of recurrence and non-recurrence according to age ≥20 (P>0.999), presence of at least one vascular risk factor (P>0.999), proportion of MMD and moyamoya syndrome (MMS) (P=0.712), location of treated vessel (P>0.999), and treatment methods (P=0.704).
DISCUSSION
The results of our study are in agreement with what is already known by medical professionals with and without previous experience with EVT for moyamoya-related intracranial steno-occlusive lesions [15]. Although a periprocedural complication rate of 6.5% in the current analysis appears relatively low, these complications must be avoided as far as possible since both instances of periprocedural complications were due to catastrophic vessel ruptures and hemorrhage with severe consequences. Furthermore, the angiographical recurrence rate of 69.2% with a short follow-up interval was unsatisfactory. These results may explain why there have been few reports regarding this issue following a previous systematic analysis from 2014 that reported similar unsatisfactory results [15].
The clinical relevance might be limited due to the potential selection bias of the patients in each report; however, we found that the occurrence of angiographical recurrence was unaffected by age, vascular risk factor, proportion of MMD or MMS, location of the treated vessel, or treatment methods. Caution must be exercised in the generalization of these results since the cases included in the reports were highly selective and neither controlled nor consecutive.
The diagnosis of MMD has been based on subjective characteristic angiographical findings. However, recent advances in the genetics of MMD enabled identification of the p.R4810K variant of the Ring finger protein 213 (RNF213) gene as the strongest susceptibility gene for MMD in East Asian people. Another study showed that RNF213 in MMD plays important roles in the appropriate gene expressions of endothelial cells in response to inflammatory signals from environments [16]. Although MMD is not an inflammatory disease, this study suggested that inflammation might have the important roles in the development of MMD. Moreover, the combination of high-resolution magnetic resonance imaging (HR-MRI) and genetic mutation can predict the disease progression or recurrent cerebrovascular events. It is suggested that plaque on HR-MRI is associated with disease progression in patients with MMD [17]. Furthermore, in patients with intracranial atherosclerosis, RNF213 gene mutation was associated with recurrent cerebrovascular events [18].
Although the effects of surgical revascularization for the prevention of hemorrhagic stroke in MMD were shown in the Japan Adult Moyamoya (JAM) trial, the efficacy of surgical revascularization for the prevention of ischemic stroke in MMD had not been determined [19]. Nevertheless, revascularization or flow-augmentation surgeries have been performed to prevent ischemic stroke, albeit with relatively higher rates of permanent neurological deficits and postoperative complications following the surgery [4]. In light of this dilemma, we might consider the potential role of EVT for the treatment of symptomatic steno-occlusive lesions in MMD. At the very least, EVT might play a role in delaying the surgical flow-augmentation for a certain period. Indeed, it is likely that the minimization of procedure-related complications such as vessel rupture could maximize the efficacy of EVT for MMD. In this study, although periprocedural complications occurred in two cases, one procedure (patient #6) was performed in a near-occluded (>99%) MCA, which was non-indicative of EVT [5]. Accordingly, if the patients are selected with the appropriate criteria, the rate of complications might be reduced to one in 31 cases (3.2%).
There are few reports on the potential role of EVT in the management of symptomatic steno-occlusive lesions in MMD. Frequent occurrence of in-stent restenosis has led to poor long-term durability and limited the efficacy of EVT as a treatment modality. To solve this problem, the use of DES could be considered. Since the effectiveness of DES has already been shown in coronary interventions, DES might lead to improvements in in-stent restenosis in MMD after EVT [20]. In our study, one case (patient #18) received stent deployment using DES, with no angiographical recurrence [12]. It appears that the treatment modality for MMD can be broadened with further investigation into the use of DES. Therefore, although there has been no evidence for EVT in patients with MMD, the efficacy and safety of EVT using DES might be maximized in patients 1) who have focal or short segmental stenosis without severe negative remodeling in HR-MRI or 2) who are suspected of MMD-atherosclerosis spectrum disease.
Our study has some limitations. First, this study included a small number of patients, with most previous reports being case reports. There were limitations in sufficient statistical analyses, consequently, the findings require further investigation in a prospective study with larger sample size. Second, most studies included in this study were not published recently; therefore, they do not reflect the current neuro-interventional devices and techniques. Third, it appeared that the pathophysiology in previous case reports was varied, and consequently, there might have been selection biases. Inclusion of the case using DES could be controversial since the location of stenosis was the ophthalmic segment of ICA, which is not a common location of stenosis in MMD.
CONCLUSION
The role of EVT in the management of symptomatic intracranial arterial steno-occlusive lesions of MMD is limited. However, inflammatory signals in MMD may be inhibited by using DES including anti-proliferative drug. Moreover, the development of genetics in MMD could lead to prediction early angiographical recurrence, consequently we could consider the necessity of early additional treatment. These recent advances in the understanding of the pathomechanisms of MMD and the recent development of neuro-interventional devices could persuade neurointerventionists to find a new endovascular approach with better stenting mechanisms.
Notes
Fund
None.
Ethics Statement
Because the study did not involve human interaction or patient data, as institution board ethical review was deemed unnecessary. Also, consent for publication is not required as the submission does not include any images or information that may identify the person.
Conflicts of Interest
The authors have no conflict of interest to declare, except that YS is an assistant editor of Neurointervention since 2019.
Author Contributions
Concept and design: DHL. Analysis and interpretation: JCR and DHL. Data collection: JCR, MHK, and DHL. Writing the article: JCR and DHL. Critical revision of the article: JCR, YHC, EJM, YJK, BSK, YS, and DHL. Statistical analysis: JCR and DHL.