Flow Diverter Treatment Using a Flow Re-Direction Endoluminal Device for Unruptured Intracranial Vertebral Artery Dissecting Aneurysm: Single-Center Case Series and Technical Considerations
Article information
Abstract
Purpose
This study aimed to evaluate the effectiveness, safety, and technical considerations of flow diverter (FD) treatment using a Flow Re-direction Endoluminal Device (FRED) for unruptured intracranial vertebral artery dissecting aneurysms (VADAs).
Materials and Methods
We conducted a retrospective study of 23 patients with unruptured intracranial VADAs who underwent FD treatment using a FRED between June 2017 and August 2021. Symptoms, imaging findings, treatment strategies, and angiographic and clinical outcomes were evaluated. Dissections were categorized according to the dominance of the VA in which they occurred: dominant VA, co-dominant VA, and non-dominant VA.
Results
All patients successfully underwent FD treatment with either a FRED (n=11) or FRED Jr. (n=12). Complete occlusion rates were 78.3% at 6-month follow-up magnetic resonance angiography and 91.3% at 12-month. There were no instances of complications, recurrence, or retreatment during a median follow-up of 20 months. Dissections occurred in the dominant VA in 3 cases (13.0%), the co-dominant VA in 13 cases (56.5%), and the non-dominant VA in 7 cases (30.4%). Intimal flap and true lumen stenosis were observed in 39.1% and 30.4% of cases, respectively. Four cases required a bilateral VA approach due to technical difficulties, all in the non-dominant VA.
Conclusion
Flow diversion treatment using a FRED for unruptured intracranial VADAs proved feasible and safe, yielding satisfactory occlusion rates. Technical challenges were more likely in lesions involving non-dominant VAs in the acute or subacute stage, mainly due to associated intraluminal lesions compromising the arterial lumen.
INTRODUCTION
Cervicocephalic artery dissection can result in ischemic stroke or subarachnoid hemorrhage, affecting young or middle-aged adults [1,2]. The rate of intracranial artery involvement from dissection is exceptionally high in Asia [3-5]. As compared to patients in Western countries, intracranial arterial dissections in Koreans occur most commonly in the posterior circulation, particularly in the vertebral artery (VA) [1,6]. Therefore, there seems to be an ethnic or genetic disposition in the development of VA dissection [7,8].
Spontaneous cervicocephalic dissection is an important cause of stroke and presents with varying lesion locations and clinical features. The VA, especially the intradural VA, is the most common location of spontaneous cervicocephalic dissection. Most cases are accompanied by an aneurysm and may lead to subsequent subarachnoid hemorrhage [6,9,10].
There are various treatment options for VA dissecting aneurysms (VADAs) [11]. If the parent artery has sufficient collaterals and can be sacrificed, parent artery occlusion can be the most definitive treatment; however, parent artery occlusion is not feasible if the dominant VA or branches like the cerebellar artery or perforator are involved [12]. Stent-assisted coiling or multiple stentings can also be an option, but there are only anecdotal case series in the literature. Although the introduction of various flow diverters (FDs) has enabled reconstructive treatments for dissections, technical issues related to outcomes were not evaluated in detail, especially in the VAs with paired anatomical structure leading to a diverse variation of branching pattern and size of the vessel lumen [13,14].
Recently, several studies have reported treatment outcomes of FDs, especially for small-diameter vessel lesions like VADAs; however, only a few reports have described the procedural difficulties and technical aspects [11,15-21]. VADAs often exhibit complex intraluminal structures due to intimal flaps and concurrent irregular stenosis, which can complicate the procedure and potentially lead to complications [1,3]. This study aimed to evaluate the effectiveness and safety of FD treatment using a Flow Re-direction Endoluminal Device (FRED) for unruptured intracranial VADAs and describe the procedural strategies used to overcome the technical challenges encountered.
MATERIALS AND METHODS
Patients
After obtaining approval from the local institutional review board, we retrospectively reviewed all patients diagnosed with an unruptured intracranial VADA at a single tertiary institution from when the FD was first used. Between June 2017 and August 2021, 27 out of 71 patients diagnosed with VADA were treated with FD (n=23), stent-assisted coiling (n=3), or stenting only (n=1), and the remaining 44 patients were followed-up without treatment because they were regarded as having a stable condition and had no rationale for treatment.
We included 23 patients who received FD treatment using a FRED stent. The treatment criteria were as follows: (1) persistent, intractable symptoms, including headache or dizziness; (2) changes in size or shape of the VADA on short-term follow-up; (3) large size, irregular/bizarre shape, or uneven enhancement on vessel wall magnetic resonance imaging (VWMRI).
We excluded those who had an incidentally found stable fusiform aneurysm.
Informed consent for this study was waived, and consent for the procedures was obtained from all patients before treatment. Presenting symptoms, comorbidities, risk factors, imaging findings, procedural details, and angiographic and clinical outcomes were collected from electronic medical records [22].
Diagnostic Criteria and Vertebral Artery Dissecting Aneurysm Characteristics
VADA was diagnosed by high-resolution VWMRI and cerebral angiography with characteristic imaging findings: fusiform aneurysm with an intimal flap or double lumen, intramural thrombus, diffuse or multifocal segmental enhancement, or true lumen steno-occlusion by pseudolumen expansion [3,9,14,23].
Acute or subacute stage VADA was defined as recent-onset VADA occurring within 3 months of symptom onset and a chronic stage after 3 months. Symptomatic VADA was characterized by headache or infarction as a complication. The location of the VADA was categorized into dominant, co-dominant, or non-dominant VA according to the dominancy of the VA (i.e., a difference of ≥1 mm between the widths of both VAs) [24]. Branch involvement was indicated when the VADA incorporated the origin of any visible artery, including the posterior inferior cerebellar artery (PICA) or medullary perforator. Aneurysm configuration, including intimal flap, true lumen stenosis, intramural thrombus, enhancing wall thickening, uneven enhancement, and thrombosed aneurysm, was evaluated in 3-dimensional rotational angiography (3DRA) and VWMRI.
Endovascular Procedure and Antiplatelet Therapy
Aneurysmal configuration and dissection extent were evaluated before the procedures using a biplane angiography system (Artis Zee Q; Siemens Healthcare) and high-resolution vessel wall MRI. All procedures were performed under general anesthesia (n=14) or local anesthesia with conscious sedation (n=9) with systemic heparinization.
A 6-F distal access guiding catheter (Codman Neuro) or 5-F intermediate catheter (MicroVention) with a 6-F long sheath (Cook Medical) was positioned in the distal V2 or V3 segment of the VA. A Headway 21/27 (MicroVention) was navigated over a 0.014-inch microwire in the target artery beyond the aneurysm. A FRED or FRED Jr. (MicroVention) was carefully deployed across the VADA. After deployment, a final angiogram was obtained to assess the stent position and periprocedural complications, including thromboembolism and hemorrhage. Flat-panel computed tomography was performed to evaluate the expansion and wall apposition of the FD.
All patients were checked for P2Y12 reaction unit and received tailored antiplatelet premedication (i.e., aspirin and clopidogrel, or low-dose prasugrel) [25-28]. During the procedure, each patient received 70 IU/kg of intravenous heparin to attain an activated clotting time of approximately 250–300 seconds. An additional 1,000 IU of heparin per hour was administered to maintain the activated clotting time. Aspirin and clopidogrel, or low-dose prasugrel, were maintained for 3–6 months, and aspirin was followed for 6–12 months, depending on the aneurysm occlusion status.
Outcome Measures and Follow-up
We routinely obtained immediate post-procedural MRI with diffusion-weighted images and time-of-flight (TOF) magnetic resonance angiography (MRA) within 24 hours after the procedure. Imaging follow-up was performed every 6 months until the aneurysm was completely occluded on TOF-MRA and at 1-year intervals after that. Digital subtraction angiography was performed when residual or recurrent lesions that required retreatment were suspected on MRA.
Angiographic outcomes were determined by 2 interventional neuroradiologists and categorized as complete (100%), near-complete (≥90%) with a small neck remnant, or incomplete (<90%). The patency of the parent artery or branch vessel was evaluated using TOF-MRA source images. “Aneurysm recurrence” was defined as an increase in the overall size of the VADA as well as an increase in the size of the contrast-filled portion within the VADA.
“Adverse event” was defined as any complication occurring during the periprocedural or follow-up period that was categorized as hemorrhagic or ischemic. Imaging findings suggestive of infarction or hemorrhage were also reported as complications regardless of the presence of symptoms. In patients with initial symptoms, whether the symptom improved after the procedure was reported. The modified Rankin scale (mRS) score was used to assess functional outcomes on each patient’s last visit to the outpatient clinic.
RESULTS
Baseline Characteristics
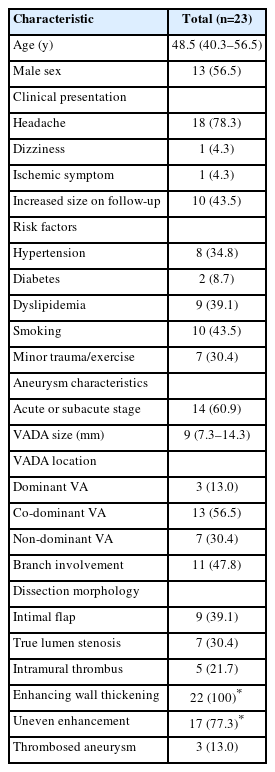
A total of 23 patients (median age, 48.5 years; 13 [56.5%] male) with an unruptured intracranial VADA were included in this study (Table 1). Eighteen patients (78.3%) had symptoms, and all had headaches. Dizziness and cerebral infarction were each present in 1 patient. The size of the VADAs increased during follow-up in 10 patients (43.5%). Regarding vascular risk factors, hypertension was found in 8 (34.8%) patients, diabetes mellitus in 2 (8.7%), dyslipidemia in 9 (39.1%), and smoking in 10 (43.5%). Five (21.7%) patients had undergone minor trauma, and 2 (8.7%) had a history of vigorous exercise before the symptom onset. Five (35.7%) patients had co-existing vascular lesions, 4 (28.6%) patients had intracranial aneurysms, and 1 (7.1%) patient had fibromuscular dysplasia.
Fourteen (60.9%) patients had acute or subacute VADAs, and 9 (39.1%) had chronic VADAs. The maximum diameter of the VADAs was 10.4 mm on average (median, 9; interquartile range, 7.3–14.3). All VADAs were in the intradural VA (V4 segment): 3 (13.0%) in dominant VA, 13 (56.5%) in co-dominant VA, and 7 (30.4%) in non-dominant VA. Eleven (47.8%) VADAs involved the origins of the PICA or perforator. Intimal flap and true lumen stenosis were observed in 9 (39.1%) patients and 7 (30.4%) patients, respectively. They were more commonly observed in the acute or subacute stage, with 8 (57.1%) patients and 6 (42.9%) patients respectively, compared to 1 (11.1%) patient for both in the chronic stage. In patients who underwent enhanced imaging, enhancing wall thickening was observed universally. Uneven enhancement was found more frequently in acute or subacute VADAs (85.7%) compared to chronic cases (62.5%). Thrombosed aneurysms, on the other hand, were exclusively identified in the chronic group, accounting for 33.3% of these cases.
Procedural Outcomes
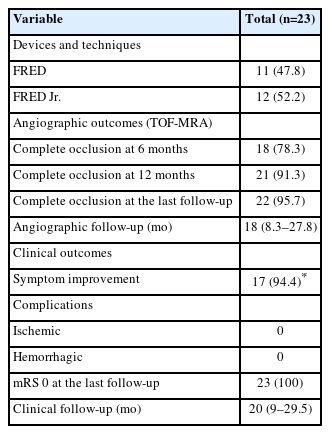
The FD treatment was carried out using either a FRED or FRED Jr. stent. In the VADAs involving the dominant VA, a FRED was used, while the FRED Jr. was employed in non-dominant VA lesions (Table 2). In cases of co-dominant VA, a FRED was deployed in 8 (61.5%) patients, and the FRED Jr. was used in 5 (38.5%) patients. Successful deployment of FDs was achieved in all cases, with each demonstrating a patent VA lumen and slower contrast inflow and stagnation in the VADA compared to the initial angiography. In 4 (17.4%) patients, technical challenges necessitated a bilateral VA approach. Notably, all of these lesions were associated with an intimal flap and stenosis in the non-dominant VA. Further, 3 of these cases were either in the acute or subacute stage of their disease course.
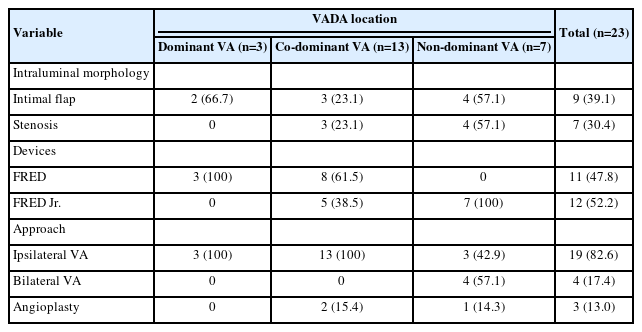
Distribution of intraluminal morphologies and corresponding treatment strategies according to the location of vertebral artery dissecting aneurysms
In 1 case with the bilateral VA approach, despite multiple attempts, the antegrade passage through the dissected segment was unachievable, leading to the FD being deployed in a retrograde manner from the contralateral VA (Fig. 1). In another case where traversing the dissected segment was challenging, a microcatheter could be introduced along a coil that smoothly passed into the PICA along the blood flow (Fig. 2). The smaller, low-profile microcatheter (0.017-inch inner diameter) used for the coil was then switched for a larger microcatheter (0.021-inch inner diameter) for FD deployment.

Flow diverter (FD) stenting via contralateral approach for patients with hypoplastic vertebral artery (VA) dissection. (A) An 11.5-mm dissecting aneurysm at the V4 segment of the non-dominant right VA involving right posterior inferior cerebellar artery (PICA) origin was noted on 3D rotational angiogram. (B) Cross-sectional 3D image of the dissecting aneurysm showed focal stenosis at the starting point of the dissection (arrow) and the intimal flap just beyond the right PICA, which precluded the passage of the guidewire into the basilar artery. (C) Sectional image across the dotted line (B) shows the intimal flap (arrow) compromising the VA lumen. (D) A microcatheter was retrogradely advanced via the left VA to avoid inadvertent passage of the guidewire into the aneurysmal pseudolumen and the right PICA when it passed from below. (E) After deployment of the FD, working segment marked as two radiopaque helical strands (arrows) of the FD (FRED) covers the dissecting aneurysm, and both ends of the FD were well opposed to the proximal and distal parent artery. (F) On the time-of-flight magnetic resonance angiographic follow-up at 14 months, the aneurysm was completely occluded with preservation of the parent artery and incorporated PICA. The stented segment of the VA and PICA origin look narrow due to magnetic susceptibility artifact artifacts caused by FD. FRED, Flow Re-direction Endoluminal Device.
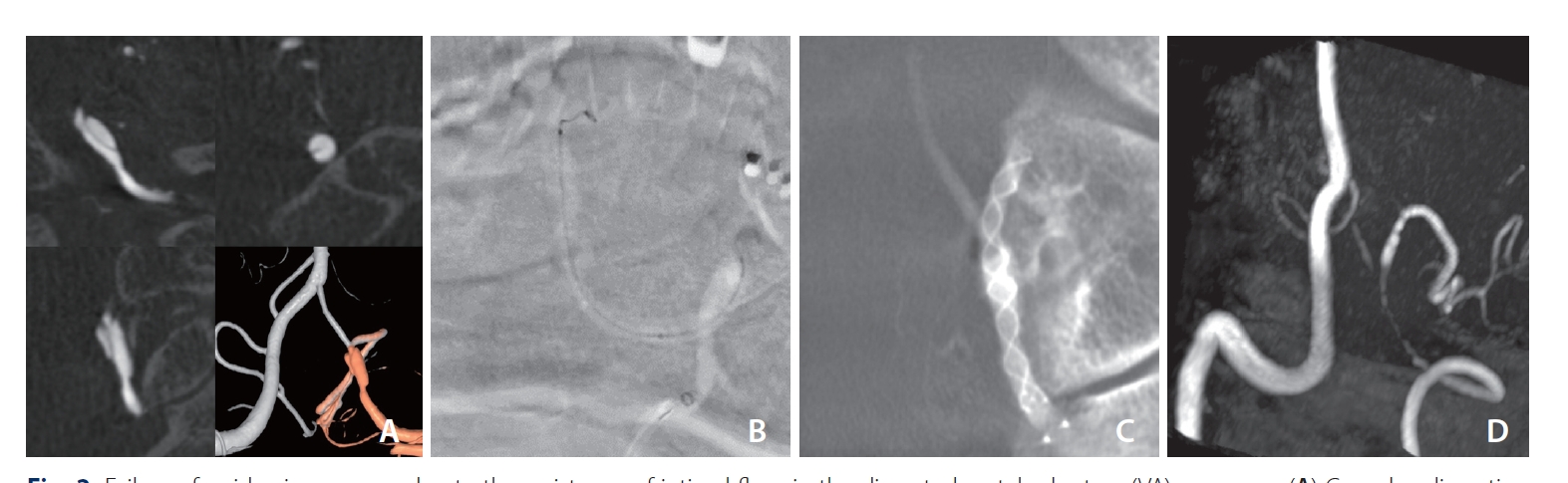
Failure of guidewire passage due to the resistance of intimal flaps in the dissected vertebral artery (VA) aneurysm (A) Complex dissecting aneurysm in the left VA just below the origin of the posteroinferior cerebellar artery (PICA). A guidewire could not pass into the left PICA because of the dissected intimal flap below the left PICA. (B) While a detachable coil was introduced into the aneurysm to sacrifice the left VA just below the left PICA, the coil passed into the left PICA and then a microcatheter was subsequently introduced into the left PICA along the detachable coil. (C) A flow diverter (FD) could be deployed along the PICA and VA. (D) The aneurysm had disappeared on the 6-month follow-up MRA, which showed good patency of the left PICA. The stented segment of the VA and proximal PICA look narrow due to magnetic susceptibility artifacts caused by FD. The distal V4 segment above PICA origin was reduced in size probably due to the flow diversion effect by the FD. MRA, magnetic resonance angiography.
Out of the 7 patients presenting with pre-existing true lumen stenosis, 3 cases required post-stenting balloon angioplasty for over 50% stenosis following FD placement. This stenosis was observed at the origin of the dissecting flap and was associated with tight angulation, implying that the parent artery stenosis, related to the underlying dissection, caused incomplete expansion of the FD.
Angiographic and Clinical Outcomes
The rate of complete occlusion was 78.3% (18 out of 23) at the 6-month follow-up MRA and increased to 91.3% (21 out of 23) at the 12-month follow-up (Table 3). Of the 2 patients with a residual neck, 1 eventually exhibited complete occlusion at the 18-month follow-up. No instances of aneurysm recurrence or retreatment were recorded during the follow-up period. Furthermore, no periprocedural or follow-up hemorrhagic or infarct strokes were observed in any of the patients. All patients showed an mRS score of 0 during a median clinical follow-up duration of 20 months.
DISCUSSION
This study showed that flow diversion treatment using a FRED for unruptured intracranial VADAs was feasible, safe, and resulted in good outcomes. Technical difficulties were primarily caused by stenosis and the intimal flap within the dissecting aneurysm. These complex intraluminal lesions were particularly common in non-dominant VAs, especially in acute or subacute VADAs.
FD treatment for posterior circulation aneurysm (e.g., saccular, dissecting, and fusiform dolichoectatic aneurysms) has been seldomly reported, and saccular and dissecting aneurysms are often associated with comparatively good outcomes [29-31]. FDs may have an advantage in stabilizing the dissection flap back against the wall and preventing further progression of VADAs, which is a segmental disease rather than a focal lesion. Recently, many studies have reported good results on FD treatment for unruptured VADA with favorable occlusion rates (56–91%) and safety outcomes (0–17%) (Table 4) [11,15-21]. Our study showed comparable results and described how to overcome technically tricky situations, especially in acute and subacute VADAs.
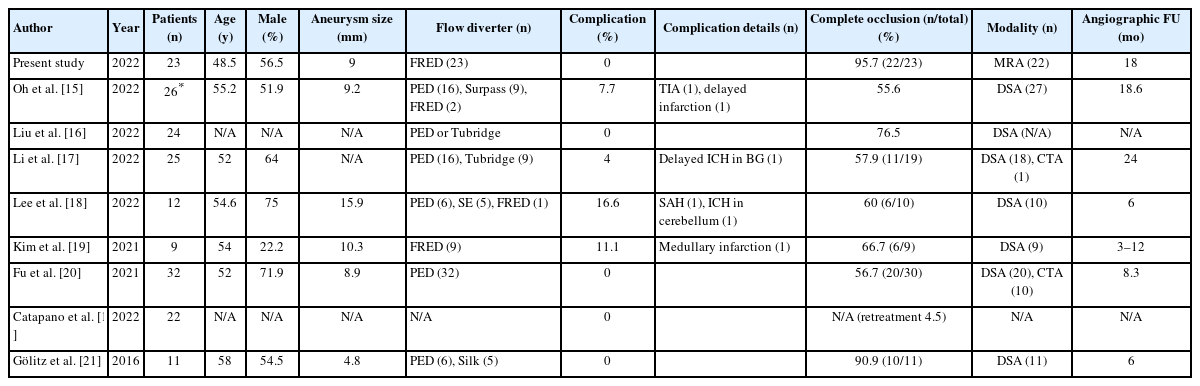
Summary of studies including flow diverter treatment of unruptured vertebral artery dissecting aneurysms
The most critical issue in using such a device is overcoming the VA configuration. The VA is an embryologically unfused artery that is a bilaterally located vascular anatomy in the embryo. The dissection may involve the dominant, co-dominant, or non-dominant VA [11]. FD treatment, which can preserve the parent artery, may be the only option to treat VADAs involving the dominant VA or major branches (i.e., PICA, medullary perforator, and anterior spinal artery) with less concern about recurrence. Even if the involved VA can be sacrificed in certain situations, preservation of the VA may be beneficial when the contralateral VA has simultaneous dissection.
In our study, the approach strategy was different depending on the concomitant intraluminal lesions that made it difficult to pass the dissection segment, such as true lumen stenosis or intimal flap, and the dominance of the VA on the affected side (Supplementary Table 1). All cases with the bilateral VA approach had concomitant intraluminal lesions in a non-dominant VA. Small profile FDs such as the FRED Jr., which is compatible with a 0.021-inch microcatheter, may have an advantage in navigating through a stenotic dissected segment or retrograde approach.
The morphological characteristic of dissecting aneurysms has been described as a fusiform or dissecting aneurysm [32,33]. Intracranial VA dissection usually has 2 main outcomes: hemorrhagic rupture with a poor prognosis or ischemic obstruction with a relatively better prognosis. Management related to the distinctive difference among dissecting aneurysms has not been described in detail. Certain types of dissection showed a fusiform configuration with varying stages of thrombus in the vessel wall, progressing rather slowly and presenting clinical problems in the chronic stage. Dissecting aneurysms related to pseudoaneurysms suddenly leads to acute symptoms like severe headaches and neck pain. Therefore, such dissecting aneurysms may be found in the acute or subacute stage with the development of a pseudoaneurysm associated with the intimal flap. In this study, acute or subacute group patients had a dissecting aneurysm in relatively small vessel diameters as in the VA. They showed distinctive symptom onset and typical dissection on high-resolution MRI and 3DRA.
This study has several limitations. First, ruptured VADAs were not included in this study, which may have different outcomes from unruptured ones. However, the use of FDs for ruptured VADAs is currently not allowed in our nation due to insurance coverage issues. Second, we could not compare the outcomes between various FDs. Further studies are required to evaluate the outcome of the FRED and FRED Jr. compared with other devices in applications for ruptured VADAs. Third, the use of TOF-MRA as the follow-up modality may present certain limitations in accurately assessing both the aneurysm and the parent artery. Moreover, there exists a possibility that TOF-MRA may overestimate the occlusion effect induced by FD treatment due to its limited ability in detecting subtle residual aneurysm fillings. However, a recent meta-analysis reported that TOF-MRA shows excellent sensitivity and specificity for aneurysm follow-up after stent-assisted coiling and FD [34]. Furthermore, nitinol-based FDs, including the FRED, are reported to have relatively few MRI artifacts compared with chromium-cobalt-based stents [35]. Therefore, we routinely used TOF-MRA for the follow-up, which does not require radiation and contrast medium, and considered it sufficient to evaluate clinically relevant residual filling or stenosis. Lastly, as this study was retrospective, there were no clear size or morphology criteria in treatment decisions, and therefore there may have been room for the operator’s subjectivity in the treatment decision.
CONCLUSION
Our study indicates that flow diversion treatment using a FRED for an unruptured intracranial VADA is feasible and safe, resulting in favorable occlusion rates. Intraluminal lesions such as intimal flap and stenosis were identified as key factors in technical difficulties, particularly in lesions involving the non-dominant VA in acute or subacute stages. A comprehensive evaluation of VADAs using high-resolution VWMRI and 3DRA is therefore paramount to anticipate these challenging situations and establish appropriate treatment strategies.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.5469/neuroint.2023.00199.
Lesion type characteristics that required a different approach according to vertebral artery dominancy
Notes
Fund
None.
Ethics Statement
This study was approved by the local Institutional Review Board (IRB No. 2020-0280), and the requirement for written informed consent was waived. This article does not include any information that may identify the person.
Conflicts of Interest
DCS has been the Editor-in-Chief of the Neurointervention since 2018. No potential conflict of interest relevant to this article was reported. YS has been the Assistant Editor of the Neurointervention since 2019. No potential conflict of interest relevant to this article was reported. No other authors have any conflict of interest to disclose.
Author Contributions
Concept and design: DCS. Analysis and interpretation: SIP and BK. Data collection: SIP and BK. Writing the article: DCS and YS. Critical revision of the article: DCS and YS. Final approval of the article: SIP, BK, DCS, and YS. Statistical analysis: YS. Overall responsibility: YS.