INTRODUCTION
Endoluminal reconstruction with a flow diverter device has emerged as a viable and often preferable alternative to traditional techniques for the treatment of intracranial aneurysms [1]. These devices, which provide higher coverage of the parent vessel (30â50%) compared to conventional intracranial stents, can decrease blood flow into the aneurysm, resulting in stasis of the intrasaccular blood products and eventual aneurysmal thrombosis. Morbidity, thromboembolic complications, and mortality associated with the treatment of unruptured aneurysms with flow diversion have been comparable with that reported in stent-assisted coiling literature [2,3].
The recent publication of the 5-year follow-up of the Pipeline for Uncoilable or Failed Aneurysms trial shows the long-term safety and efficacy (specifically, a progressive aneurysm occlusion rate over years without adding complications) of the first generation Pipeline Embolization device [4]. Although the only flow diversion device with Food and Drug Administration approval is the pipeline embolization device [5], some other devices are available in Europe and in US clinical trials.
Here we present the first clinical usage of the largest flow diverter available, the 6Ã50 mm DERIVO embolization device (DED; Acandis GmbH & Co. KG, Pforzheim, Germany), into the arterial circulation for a cervical internal carotid artery endovascular reconstruction.
CASE REPORT
A patient in his/her sixties experienced pain in the right upper jaw and vomited repeatedly, then felt an ipsilateral headache. Three days later, he/she had difficulty swallowing and could not drink or talk properly. At admission, a neurological examination revealed right lower cranial nerves (IXâXII) palsy (Collet-Sicard syndrome) without any pyramidal or cerebral signs. The patient was normotensive.
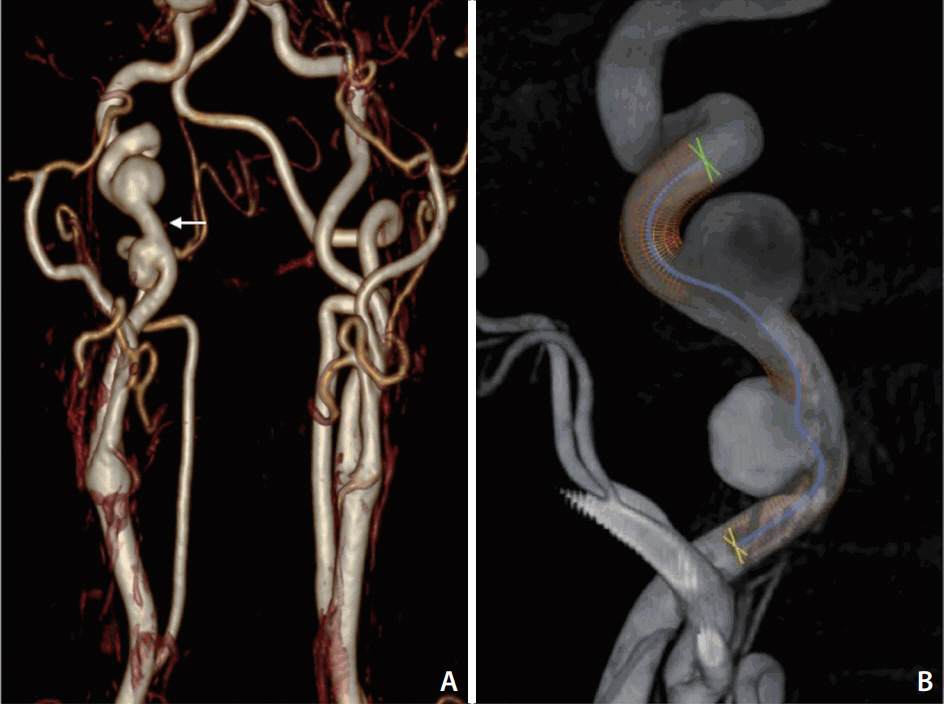
Magnetic resonance angiography and subsequent angiography demonstrated 2 aneurysms/pseudoaneruysms at the right cervical carotid artery associated with tortuosity, probably in the context of a cervical dissection (Fig. 1). Considering the location and size of the aneurysms, as well as the patientâs age, endovascular treatment with a stent was recommended. A 6Ã50 mm DERIVO embolization device (Acandis GmbH & Co. KG, Pforzheim, Germany) was selected according to the parent artery maximum diameter (6 mm) (Fig. 1).
Plavix 75 mg/day and aspirin 100 mg/day were given daily from 5 days prior to the intervention. The procedure was performed under general anesthesia. Heparin was administered at the beginning of the procedure and intermittently during the procedure to maintain an activated clotting time between 250 and 290 seconds.
A triaxial system into the right cervical internal carotid artery (ICA) was built with a Navien 072 guide catheter (Medtronic Neurovascular, Irvine, California, USA) through a 7 F envoy guiding catheter and a Neuroslider-027 microcatheter (Acandis GmbH & Co. KG).
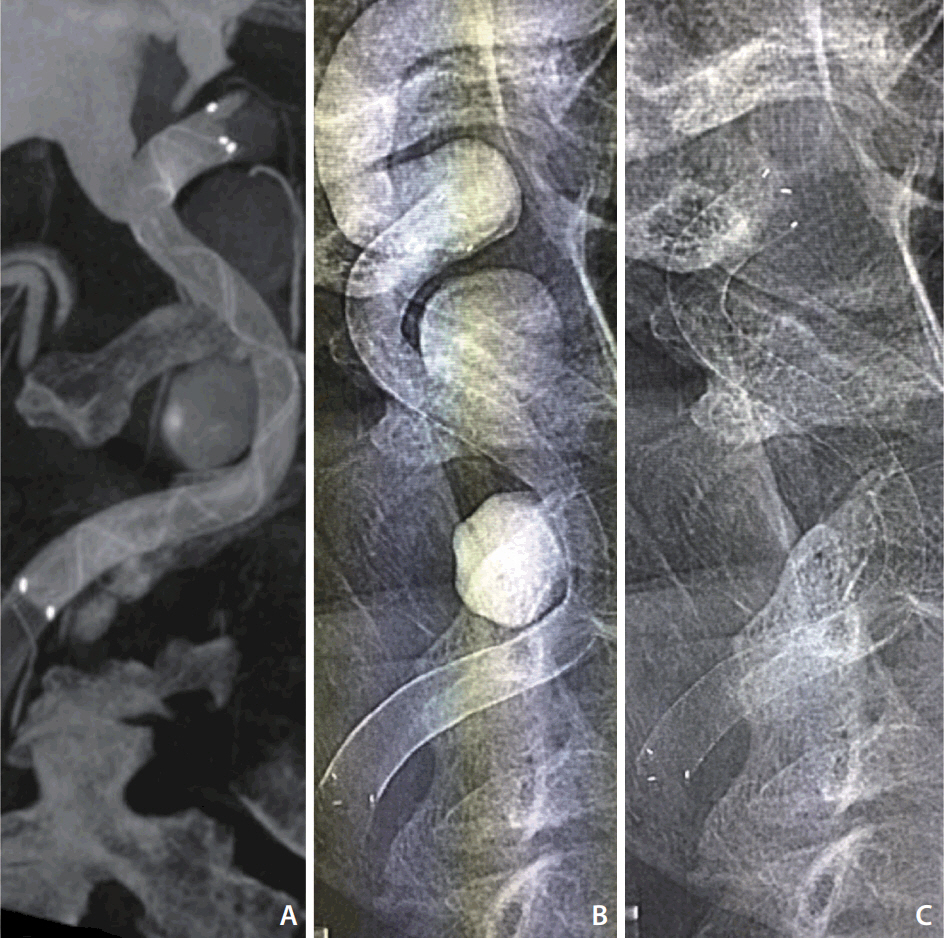
The 6Ã50 mm DED was deployed through the Neuroslider-027 microcatheter across the neck of the aneurysms. Despite the deployment starting at the landing zone, neither intermediate/guiding catheter were kicked-back, providing complete coverage of the aneurysms and artery reconstruction (Fig. 2).
Technically, once the distal-end opened, we deployed the device using push/pull maneuvers as learned from our experience with smaller DERIVO devices (during the first-third of the device opening, the delivery force was 50% push and 50% pull in order to avoid proximal migration, then 30% push and 70% pull until complete deployment). Contrast stagnation of both aneurysms was achieved with a single device, so a second device deployment was not considered (Fig. 3). The patient had an uneventful postoperative course and was discharged to go home on postoperative day 3.
DISCUSSION
Morbidity, thromboembolic complications, and mortality associated with the treatment of unruptured aneurysms with flow diversion have been comparable with that reported in stent-assisted coiling literature [1]. Poor wall-apposition has been described as a predictive factor for immediate instent and delayed thrombosis after stents use [6]. In this sense, precise measurement and device selection are mandatory steps when considering flow diverter usage in order to avoid potential complications [7,8].
One significant usage limitation of flow diverters is the parent artery diameter, especially at the posterior circulation or cervical segments, since the maximum opening of the sizes available are recommended for vessel diameters between 5.2â5.75 mm. In arteries measuring more than 5.75 mm, other endovascular techniques, such as telescoped stents could be considered [9] instead of current flow diverters.
The DED is a flow diverter consisting of 24 wires that are looped at the distal end, and so result in a braid of 48 wires. All wires are nitinol with a radiopaque platinum core. A 27-microcatheter is required for the delivery of the device. The DED is available in lengths between 15 and 50 mm and diameters between 3.5 and 6 mm. It has closed distal ends and a flaring of 25 to the outside at both ends for secure wall apposition immediately after the initial distal opening. The device can be safely recaptured before the point of no return and repositioned if an adjustment and superior placement is needed. In the current version of the DED, all nitinol wires feature an opaque platinum core in addition to the radiograph markers on both ends making the contour of the device clearly visible under fluoroscopy (Fig. 3).
In our case, despite the size and length, no learning curve was needed. The device fully opened from the beginning, and the deployment technique did not differ from smaller DERIVO devices as mentioned above.
In the present case, although there could be argument regarding the necessity of any treatment for cervical ICA aneurysms, we decided to treat the lesion since the lesion was symptomatic. The advantage of the DERIVO device lies in the availability of a larger diameter compared to other flow diverter devices, which would not fit in this kind of large artery lesion.
The 6 mm device opens until 6.2 mm, which may be useful for some large or fusiform aneurysms especially located in the vertebrobasilar system or extracranial arterial segments. Also, the availability of the 50 mm version allows the operators to minimize the use of multiple telescoped devices, which can then lower the potential thromboembolic complications [10] of the flow diverter.
The presented 6Ã50 mm device expands the subgroup of patients who can benefit from flow diversion technology. We are reporting this case with a view to adding one more option to our arsenal of aneurysm endovascular treatment.
Precise measurement and device selection are mandatory for flow diversion in order to avoid potential complications. One significant usage limitation of flow diverters is the parent artery diameter, especially at the posterior circulation or cervical segments. Larger devices, like the DERVIO 6Ã50 mm, could expand the subgroup of patients who can benefit from flow diversion technology.