 |
 |
- Search
| Neurointervention > Volume 15(3); 2020 > Article |
|
Abstract
Purpose
Four key bench-top tests, including trackability, conformability, wall-apposition, and bending stiffness, were performed to understand the mechanical characteristics in 3 different types of stents applicable for treatment of intracranial atherosclerotic stenosis: Balloon-expandable D+Storm, Pro-Kinetic Energy, and self-expandable Wingspan stents.
Materials and Methods
Trackability was assessed by measuring the tracking forces of each stent with its delivery systems. Conformability and wall apposition were quantified and analyzed using curved vessel models. A 3-point bending test was employed to evaluate bending stiffness.
Results
D+Storm showed the lowest tracking forces while the conformability of the Wingspan stent was superior to that of the tested stents. Pro-Kinetic Energy and D+Storm had better wall apposition in curved vessels than the Wingspan stent. Bending stiffness of the Wingspan stent was notably lower, whereas no significant differences were found between D+Storm and Energy. Pro-Kinetic Energy and D+Storm not only indicated lower gap ratios between the struts and the vessel wall but also maintained good wall apposition even in the curved model.
Stroke is one of the leading causes of death worldwide, and ischemic stroke is made up of 87 percent of stroke cases [1-3]. Especially, atherosclerotic stenosis of the intracranial arteries plays a significant role and is associated with a high risk of ischemic stroke [4,5]. To treat intracranial atherosclerotic stenosis (ICAS), medical therapies such as antiplatelet and antithrombotic agents have been applied as primary options [6-8]. However, it is often reported that many patients with ICAS who received traditional medicine treatment had experienced recurrent transient ischemic attacks (TIAs) and stroke [9,10]. As an alternative approach to such treatment, there has been recent attention on endovascular techniques to reduce the incidence of TIAs and stroke [11]. In particular, the intracranial stenting has become a commonly accepted treatment location for patients with ICAS, as corroborated by several studies reporting that stent placement for atherosclerotic stenosis is feasible and effective [12-14].
In the early stage of endovascular treatment using intracranial stenting, balloon-expandable stents (BES) are the only available option for intracranial atherosclerotic stenosis, which is intended to be designed for treating cardiovascular disease [15]. However, there are several drawbacks in using balloon-expanding stents, such as low flexibility, difficult delivery in tortuous vessels, and risk of vessel injury due to over-expansion [16]. To overcome these limitations of the BES, the self-expanding stent such as the Wingspan stent was introduced in 2008 specifically to treat ICAS due to its better flexibility, which facilitates the delivery through cerebral arterial anatomy [17,18]. Nonetheless, recent studies reported that applying the Wingspan stent system with intracranial stenosis is not superior to balloon-expanding stents, and the stent performance, feasibility, and effectiveness of the Wingspan compared to the BES remains unclear and controversial [19].
In this study, we conducted an in vitro benchtop comparison to evaluate the mechanical features of 3 different types of stents that are currently widely used for the endovascular treatment of ICAS. The first is the self-expandable stent Wingspan (Boston Scientific, Natick, MA, USA). The second and third are balloon-expandable stents, PRO-Kinetic Energy (Biotronik, Bulach, Switzerland) and D+Storm (CGBio, Seoul, Korea). The wingspan stent system consists of a self-expandable nitinol stent in combination with the Gateway angioplasty balloon, designed for the treatment of ICAS. The stent has a similar design platform with zig-zag struts and opencell design of Neuroform stents used for treatment of a wider-necked intracranial aneurysm. The only main difference is that the Wingspan stent has a wider and thicker strut dimension than that of Neuroform. The Pro-Kinetic Energy and D+Storm stent systems involve balloon expanding cobalt chromium stents. The Pro-Kinetic Energy stent is noted for its ultrathin strut (60 ╬╝m) and double helix strut design that allows high flexibility and deliverability. Another balloon-expandable stent D+ Storm (newly released from CGBio Co. Ltd.) has S-symmetric waved struts and a 6&8 open-cell design. The goal of the study is to gain a better understanding of the mechanical behavior of 3 different intracranial atherosclerotic stenosis stents through various benchtop assessments including trackability, conformability, wall-apposition, and bending stiffness.
Three intracranial stenosis stents, Wingspan (15 mm length; Boston Scientific), PRO-Kinetic Energy (15 mm length; Biotronik), and D+Storm (16 mm length; CGBio), were investigated for benchtop comparative tests in the study. All stent models were uncovered metallic stents with a diameter of 3.5 mm. Stent material, strut dimensions, stent type, and structure analysis of the tested stent samples are summarized in Table 1. Three samples for each benchtop test were performed to calculate the average values for the mechanical properties.
The trackability describes the ability of the stent and its delivery system to be delivered softly to the target lesion through a tortuous vessel anatomy. Trackability of the stent and its system was evaluated using interventional device testing equipment (IDTE-3000; MSI, Flagstaff, AZ, USA) as shown in Fig. 1. Analysis of trackability was performed by measuring the generated track force while the crimped stent-catheter system over a guidewire (0.014ŌĆØ) was moved towards through the curved vessel model (HE-PE tubing, I.D. 3 mm) at a rate of 25 cm/min. The total track distance was 18 cm, and the measurements were performed 3 times in each stent model.
Conformability played a major role in determining the degree of geometric changes in intracranial arteries when stents were deployed. To investigate the ability of stents in conforming to their original vessels, a benchtop test of conformability was performed. Conformability was assessed by quantifying changes in angle values of a curved vessel model after stent deployment when compared to the angle value in the natural shape as seen in Fig. 2. The inner diameter 3.5mm of the customized Polyether block amide tube was used as a vessel model for the conformability test. Stents were deployed at the midsection of an approximately 9.5 mm radius curve in the vessel model. The changes in the curved vessel model pre and post deployment were photographed using a digital camera. The vessel model was placed with fixation pins except for the curvature part for angle variation in order to improve the accuracy of the angle variation post stent deployment. ImageJ software (NIH, Bethesda, MD, USA) was utilized to measure the angle variation. For each stent model, the angle value of the 3 samples was obtained to calculate the average angle variation.
Wall-apposition addresses a stentŌĆÖs ability to maintain strut apposition to the vessel wall when deployed in curved vessels. A silicone curved vessel model (Circle of Willis Model; United Biologics, Santa Ana, CA, USA) with a 3.5 mm inner diameter was used in this study, and the stents were deployed in a curved segment having a radius with a curvature of approximately 3 mm. During the test, the vessel model was filled with water containing lubricant, and the solution was kept flowing through a flow pump system (FlowTek 125 System; United Biologics). Stent deployment was undertaken 3 times in each stent model including Wingspan, PRO-Kinetic Energy, and D+Storm stents. Images were photographed to analyze the apposition of each stent model to the curved vessel wall, and ImageJ software (NIH) was used to measure the gap between the vessel wall and the deployed stents. The measurements were calibrated by using a 3.5 mm inner diameter of the vessel model as a reference. The gap ratio was measured by the gap distance divided by the diameter of the vessel.
Bending stiffness is a measure of the stent platformŌĆÖs resistance to bending deformation which relates to its flexibility. To characterize the bending stiffness, a 3-point bending test was applied in the study. The bending force of the 3-point bending test was evaluated by using a universal tensile-compression machine with its apparatus. Forces were measured with 5.0 N load cells at a speed of 10 mm/min. The measurement of bending force was performed in the expanded state as demonstrated in Fig. 3. The span length was 11 mm, and 2.2 mm of deflection (displacement) was employed according to ASTM F2606-08.
Fig. 4 and Table 2 below show the evaluation results of the bench test for trackability of the 3 different stent products. The graph of Fig. 4 shows that the overall track force of Wingspan stents was the highest, and the track force values of D+Strom and Energy stents were similar to each other. The average max track force was 0.525┬▒0.021 N for D+Storm stents, 0.625┬▒0.020 N for Pro-Kinetics Energy stents, and 1.186┬▒0.321 N for Wingspan stents. The stent expanding method of Wingspan stents (self-expandable) is different from that of D+Storm and Energy stents (balloon-expandable), and as a result, they employ different types of delivery systems. The results of the trackability bench test demonstrated that when compared to Wingspan and Pro-kinetic Energy stents, D+Strom stents showed better trackability performance.
Fig. 5 and Table 2 show the results of the conformability test. In this experiment, Wingspan stents showed the lowest angle variation value (8.78┬▒0.59), and Energy stents showed the highest angle variation value (11.80┬▒0.96). The results of this conformability bench test demonstrated that Wingspan stents had the least straightening effect on the blood vessel after stent placement and showed better performance in maintaining the original shape of the blood vessel.
Fig. 6 exhibits captured images of stents that were deployed inside the curved blood vessels for each model, and Table 2 summarizes the gap ratio values between the strut and the inner vessel wall of the model. The results of the wall apposition test showed near-complete stent apposition to the vessel wall was observed in all 12 stents after they were inserted into the curved blood vessels as shown in Fig. 6. Wingspan stents showed a tendency to maintain good wall apposition, but all stents in Wingspan tested showed severe kinking as shown in Fig. 7. On the other hand, little kinking was observed in the D+Strom and Pro-Kinetic Energy stents under the experimental curve model conditions, and only minor kinking was observed in the middle section of the curved blood vessels. The gap ratio of Wingspan stents was highest at about 12.66% among the stents tested, and the gap ratios of D+Strom and Pro-Kinetic Energy stents for stent wall apposition were 9.28% and 8.82%, respectively.
Fig. 8 and Table 2 shows bending stiffness measurements of the 3 different stents. Similar to the conformability results, Wingspan stents had the lowest bending stiffness (0.047 N), followed by D+Strom (0.084 N) and Pro-Kinetic Energy stents (0.100 N). This result shows that stents with lower bending stiffness tend to conform better to curved blood vessels. However, the bending stiffness value of Wingspan stents was significantly lower (more than 2 times lower than that of Energy stents) than that of the other 2 stents tested, suggesting that the properties of the nitinol material used for Wingspan stents would have influenced their bending stiffness.
Mechanical properties of stents are crucial in evaluating the clinical performance of in-stent treatment for intracranial atherosclerotic stenosis. Currently, there are 2 types of intracranial stenosis stents available. One is a self-expanding stent made of nitinol materials, and the other is a cobalt-chromium-based balloon-expanding stent. Clinical studies have shown pros and cons for patients treated with balloon-expanding and self-expanding stents for ICAS [20,21]. Self-expanding stents are likely to be less rigid and more flexible than balloon-expanding stents, indicating that they are beneficial for areas with more tortuous vessels as well as those with less calcified lesions. Balloon-expanding stents have lower flexibility, but higher radial force than self-expanding stents. Hence, they are beneficial in areas with increased calcified lesions [22]. To our knowledge, bench-top comparison reports on mechanical properties of stents being used for ICAS treatment are rare. Thus, it might be assumed that interventionists tend to choose the stents depending on their clinical experience rather than on their mechanical performance characteristics.
In our study, D+Storm showed the lowest tracking forces, while the conformability of the Wingspan stent was superior to that of the tested stents. Pro-Kinetic Energy and D+Storm had better wall apposition in curved vessels than the Wingspan stent. Bending stiffness of the Wingspan stent was notably lower, whereas no significant differences were found between D+Storm and Energy. Pro-Kinetic Energy and D+Storm not only indicated lower gap ratios between the struts and the vessel wall but also maintained good wall apposition even in the curved model.
The evaluation of trackability is an essential aspect in determining the proper intracranial stenosis stent, as they must go through tortuous vascular anatomy in the implantation process. In the present study, the trackability of self-expanding Wingspan and 2 different BES systems (D+Storm, Pro-kinetic Energy) were assessed by measuring track force while advancing along the pathway of the vessel model. The trackability relies on varied parameters such as the crimped cross profile of the stent, stent platform design, strut dimensions, and stiffness of the delivery system. The investigations of trackability demonstrate that D+Storm stent-catheter systems have the lowest track force among the examined stents, indicating they can provide easier delivery to the target lesion compared to Pro-Kinetic Energy and Wingspan stent systems. In contrast, the Wingspan stent exhibits a higher tracking force than both the Pro-Kinetic Energy and D+Storm stent systems.
The result of a higher track force in the Wingspan stent system was unexpected because self-expanding stent systems commonly are considered better at stent deliverability, due to not only their raw materials but also their thinner strut dimensions. This phenomenon might be attributed to a difference in the stent delivery system applied under bench-top experimental conditions when compared to the other 2 stents employing the balloon-expandable delivery system. The Pro-Kinetic Energy and D+Storm stent systems consist only of stents pre-mounted on its balloon catheter. In contrast, the Wingspan stent system has a more complex delivery system containing a pre-loaded stent, a microcatheter for stent delivery, and an additional Gateway angioplasty balloon combined together for pre-dilating the lesion. Therefore, the microcatheter with the balloon catheter system of the Wingspan stent may result in recording a higher track force when compared to the other 2 balloon catheter systems. However, further studies are needed to understand these tracking force behaviors, since clinical conditions and scenarios are much more complicated and hostile than those of bench-top experimental conditions.
Conformability is an important factor that affects the mechanical properties of intracranial stenosis stents. In particular, it may be closely associated with bending stiffness [23]. Stents usually tend to straighten out the curved vessel when they are deployed because of their stiffness. It is well known that the recurrence of stenosis (restenosis) increases as the degree of changes in the angulation of the vessel increases [24,25]. From the result of the conformability test, it appears that the Wingspan stents have better conformability as evidenced by the least amount of changes in the vessel angulation after stent placement.
In regards to flexibility, bending stiffness was examined using 3 point-bending methods. The results of bending stiffness tests also showed a similar tendency as shown in the conformability test in which the Wingspan stent had the least amount of changes in bending stiffness, followed by D+Storm and Pro-Kinetic Energy. Here, it should be emphasized that different types of stent materials play a key role in determining their conformability as well as flexibility.
Wall apposition is the ability of the stent struts to sustain attachment to the vessel walls when deployed in curved and tortuous cerebral vessels. It is obvious that incomplete stent apposition leads to adverse clinical events such as late stent thrombosis, early/delayed stent migrations, and related vessel occlusions [26,27]. Pro-Kinetic Energy and D+Storm stents are superior to the stent-vessel apposition of the Wingspan stent. All experimented stent samples were kinked at the middle of the inner curve segments, which may be attributed to the relatively small radius curvature silicone model. However, Pro-Kinetic Energy and D+Storm not only indicated lower gap ratios between the struts and the vessel wall but also maintained good wall apposition even in the curved model.
Although a mechanical comparison of the 3 different stents using bench-top measurements furthered our understanding of particular stents, the bench tests carried out in our study have several limitations. Namely, the conditions set up in our experiment do not fully reflect those in actual clinical performance. The results in clinical trials can change in vessel properties with diameters as well as tortuosity, since each stent model and type may have different mechanical strength and characteristics depending on stent diameters and length. Also, the surface friction of the tested vessel models is not identical to that of a human vessel, which may influence the apposition behavior of the tested stents. Lastly, the vessel tortuosity under bench-top conditions is less tortuous than in actual intracranial vascular arteries. Thus, further bench-top tests are needed to understand the effects of different types of stents in various experimental conditions. Despite these limitations, the bench-top results of this study can provide clinicians with useful insights on choosing the appropriate stent system for the treatment of intracranial atherosclerosis.
As demonstrated in the study results, Wingspan stents are better in conformability due to not only the lowest angle variation but also the lowest bending stiffness. However, the results of wall-apposition tests indicate that they perform the largest separation of the struts such as kinking and incomplete expansions from the curved-vessel walls, which might be explained by their inferior bending force compared to D+Storm and Pro-Kinetic Energy stents.
However, there is still a limited amount of bench-top studies on current intracranial stenosis stents to better understand their mechanical properties, particularly those that are related to clinical implications. Therefore, further investigation is required, which will facilitate in selecting the most appropriate stent system for certain target sites as well as lesion types.
Notes
Fund
This study was supported by the Korean Society of Interventional Neuroradiology (KSIN) research grant 2018.
Fig.┬Ā1.
Vessel model and stent system for trackability tests: (A) D+Storm and Pro-Kinetic Energy stent system, and (B) Wingspan stent system.
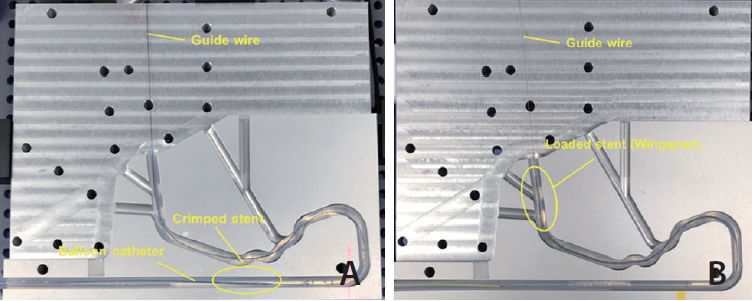
Fig.┬Ā2.
Schematic images of angle variations of the tested stents (A) before and (B) after stent deployment in the curved segment.
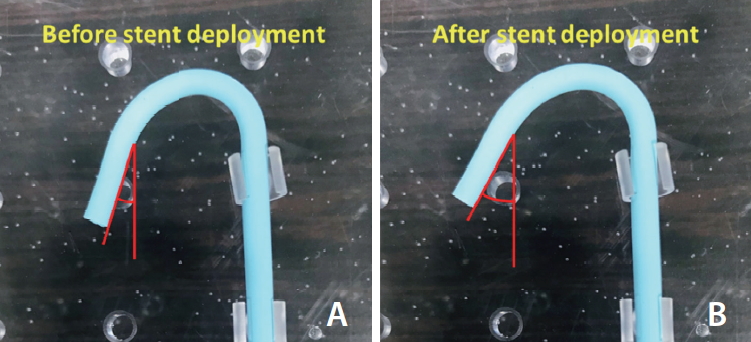
Fig.┬Ā4.
Tracking force-distance curves of trackability measurements: (A) D+Storm, (B) Pro-Kinetic Energy, and (C) Wingspan.
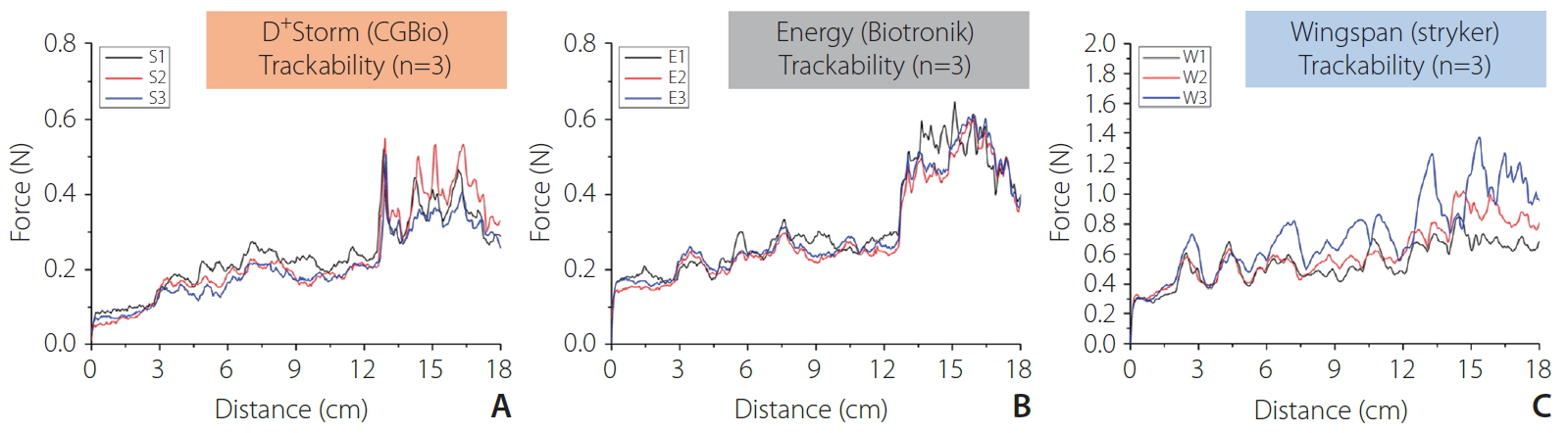
Fig.┬Ā5.
Images of angle variation results for measurements of conformability (in the following order): D+Storm, Pro-Kinetic Energy, and Wingspan.
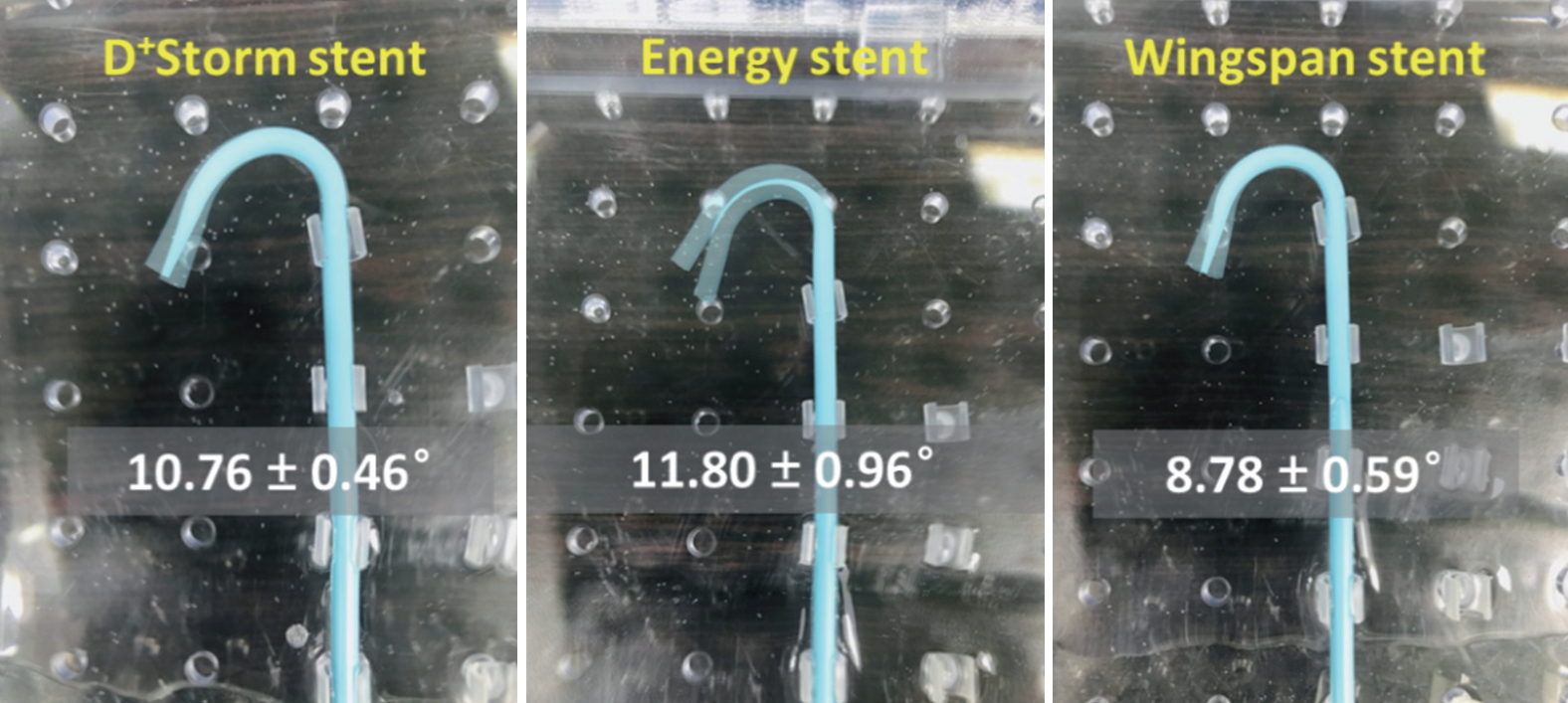
Fig.┬Ā6.
Images of stent-vessel wall apposition measurements (D+Storm, Pro-Kinetic Energy, and Wingspan stents, respectively).

Fig.┬Ā7.
Images of apposed stents to the curved vessel wall: D+Storm, Pro-Kinetic Energy, and Wingspan.

Fig.┬Ā8.
Graphs displaying bending force-displacement curves for measurement of bending stiffness: (A) D+Storm, (B) Pro-Kinetic Energy, and (C) Wingspan.

Table┬Ā1.
Stent material, strut dimensions, stent, type, and structure analysis of the tested stents (Pro-Kinetic Energy, D+Storm, and Wingspan)
Table┬Ā2.
Summary of the bench-top testing results of the tested stents: D+Storm, Pro-Kinetic Energy, and Wingspan
REFERENCES
1. Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved? Lancet Neurol 2007;6:94-97.


2. Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182-187.


3. Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259-268.


4. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396-2399.


6. Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology 2000;55:490-497.


7. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology 1995;45:1488-1493.


8. Luo J, Wang T, Gao P, Krings T, Jiao L. Endovascular treatment of intracranial atherosclerotic stenosis: current debates and future prospects. Front Neurol 2018;9:666



9. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al, Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305-1316.


10. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555-563.


11. Turan TN, Derdeyn CP, Fiorella D, Chimowitz MI. Treatment of atherosclerotic intracranial arterial stenosis. Stroke 2009;40:2257-2261.



12. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Angioplasty and stent placement in intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol 2002;23:430-436.


13. Mori T, Kazita K, Mori K. Cerebral angioplasty and stenting for intracranial vertebral atherosclerotic stenosis. AJNR Am J Neuroradiol 1999;20:787-789.


14. SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): study results. Stroke 2004;35:1388-1392.


15. Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med 1996;334:561-566.


16. Vajda Z, Miloslavski E, G├╝the T, Schmid E, Schul C, Albes G, et al. Treatment of intracranial atherosclerotic arterial stenoses with a balloon-expandable cobalt chromium stent (Coroflex Blue): procedural safety, efficacy, and midterm patency. Neuroradiology 2010;52:645-651.



17. Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007;38:1531-1537.


18. Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, et al, NIH Multi-center Wingspan Intracranial Stent Registry Study Group. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology 2008;70:1518-1524.



19. Albuquerque FC, Levy EI, Turk AS, Niemann DB, Aagaard-Kienitz B, Pride GL Jr, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery 2008;63:23-27 discussion 27-28



20. Miao Z, Zhang Y, Shuai J, Jiang C, Zhu Q, Chen K, et al, Study Group of Registry Study of Stenting for Symptomatic Intracranial Artery Stenosis in China. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 2015;46:2822-2829.


21. Liu L, Zhao X, Mo D, Ma N, Gao F, Miao Z. Stenting for symptomatic intracranial vertebrobasilar artery stenosis: 30-day results in a high-volume stroke center. Clin Neurol Neurosurg 2016;143:132-138.


22. Rohde S, Seckinger J, H├żhnel S, Ringleb PA, Bendszus M, Hartmann M. Stent design lowers angiographic but not clinical adverse events in stenting of symptomatic intracranial stenosis - results of a single center study with 100 consecutive patients. Int J Stroke 2013;8:87-94.


23. Wang Q, Fang G, Zhao Y, Wang G, Cai T. Computational and experimental investigation into mechanical performances of Poly-L-Lactide Acid (PLLA) coronary stents. J Mech Behav Biomed Mater 2017;65:415-427.


24. du Mesnil de Rochemont R, Yan B, Zanella FE, R├╝fenacht DA, Berkefeld J. Conformability of balloon-expandable stents to the carotid siphon: an in vitro study. AJNR Am J Neuroradiol 2006;27:324-326.


25. Gao B, Baharoglu MI, Cohen AD, Malek AM. Stent-assisted coiling of intracranial bifurcation aneurysms leads to immediate and delayed intracranial vascular angle remodeling. AJNR Am J Neuroradiol 2012;33:649-654.



- TOOLS
-
METRICS

-
- 5 Crossref
- 5,035 View
- 199 Download
- Related articles in NI







