|
 |
- Search
| Neurointervention > Volume 17(3); 2022 > Article |
|
Abstract
Purpose
Materials and Methods
Results
Notes
Fund
This work was supported by a grant from the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2018R1A2B6003143).
Ethics Statement
This study was approved by the Institutional Review Board at Asan Medical Center (IRB no. 2021-1102). Waiver of informed consent to participate was approved by the Institutional Review Board at Asan Medical Center (IRB no. 2021-1102) due to minimal risk to subjects and the retrospective nature of this study. De-identification of described cases and figures was performed. All patients whose cases or figures were demonstrated in this study consented to publish verbally and provided us with written consent for publication.
Conflicts of Interest
DCS has been the Editor-in-Chief of the Neurointervention since 2018. No potential conflict of interest relevant to this article was reported. YS has been the Assistant Editor of the Neurointervention since 2019. No potential conflict of interest relevant to this article was reported. No other authors have any conflict of interest to disclose.
Author Contributions
Concept and design: DCS. Analysis and interpretation: BK, YHC, and YS. Data collection: BK, YHC, and DCS. Writing the article: BK and YS. Critical revision of the article: BK, YS, and DCS. Final approval of the article: DCS. Statistical analysis: BK and YHC. Obtained funding: DCS. Overall responsibility: DCS.
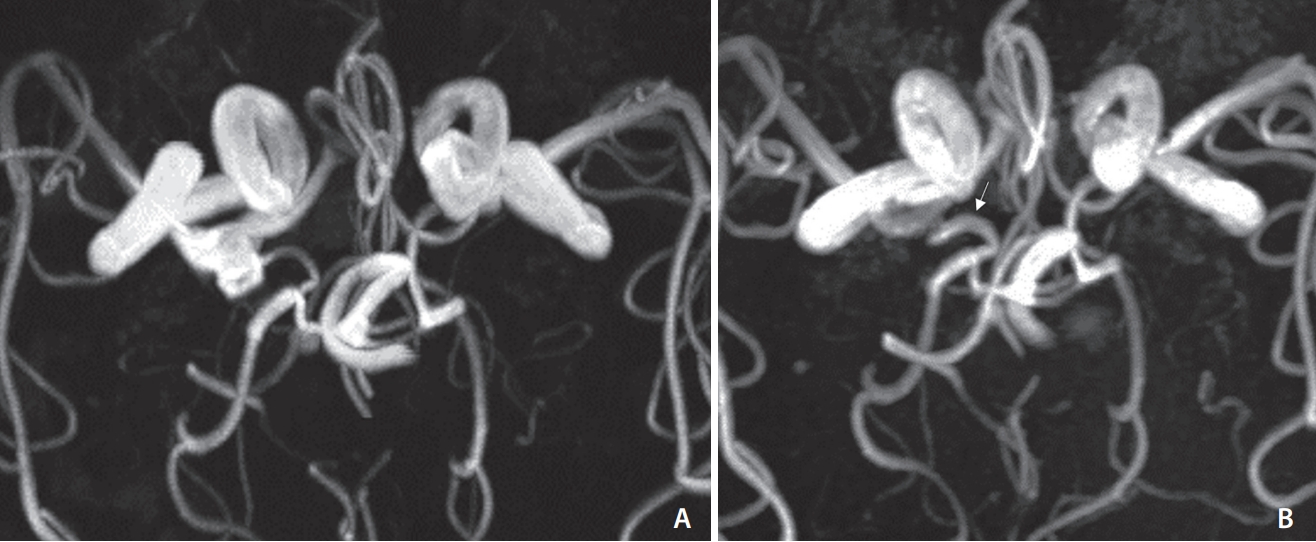
Fig.┬Ā1.

Fig.┬Ā2.
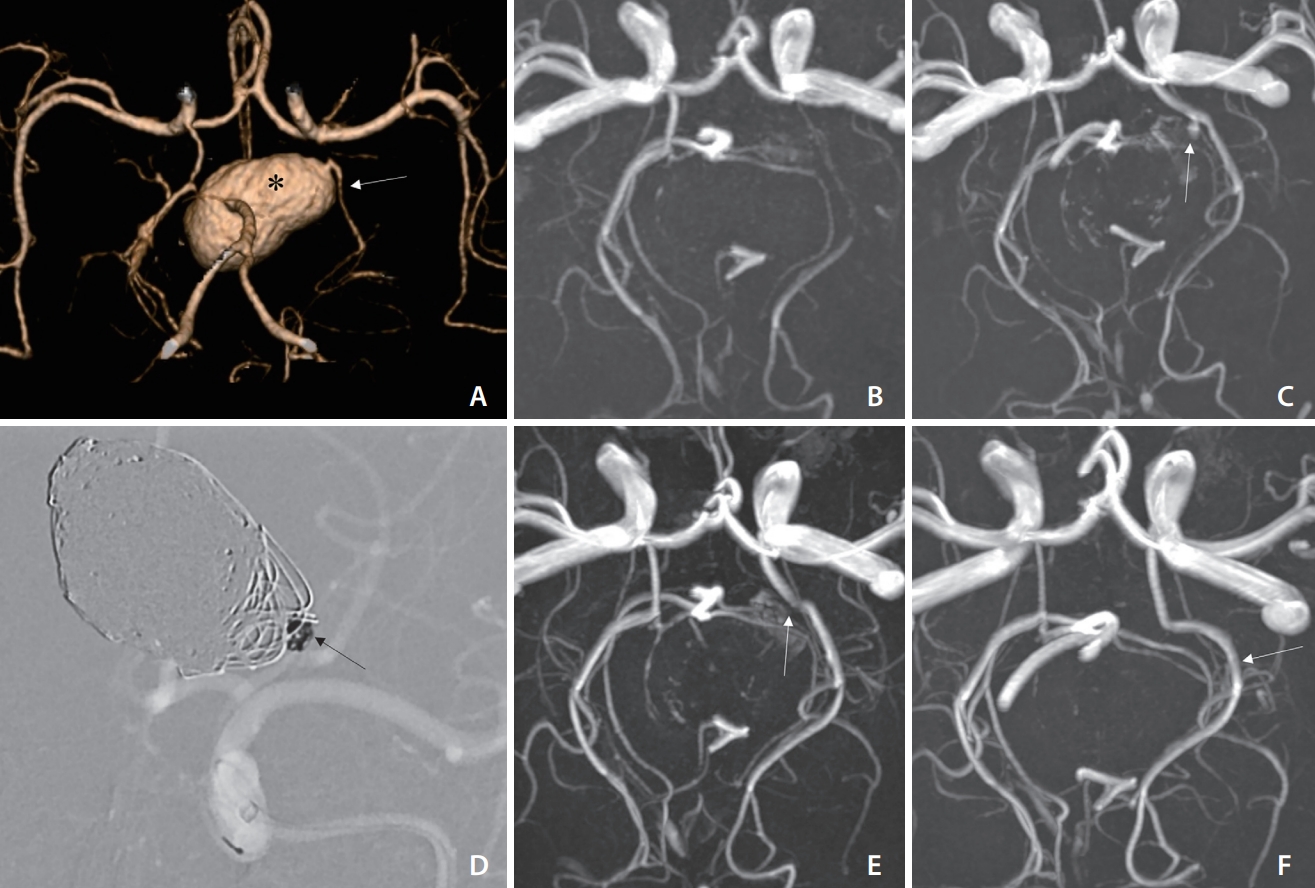
Fig.┬Ā3.
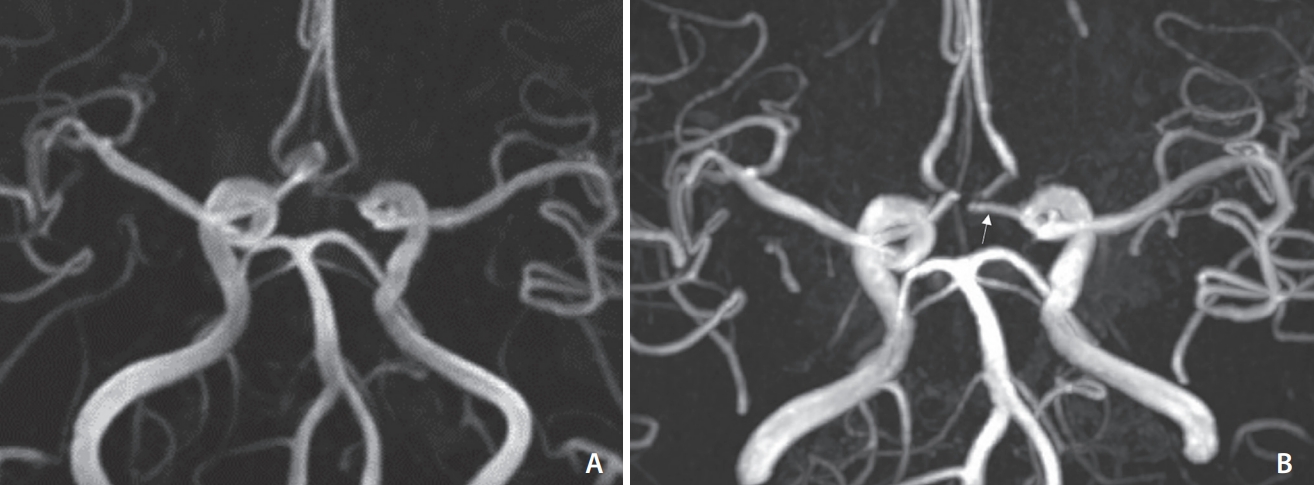
Fig.┬Ā4.