 |
 |
- Search
| Neurointervention > Volume 19(1); 2024 > Article |
|
Abstract
Purpose
Materials and Methods
Results
Notes
Ethics Statement
Ethical approval obtained from the local institutional review board (243/2021); the board waived the need for patient consent. The paper does not include any images or information that may identify the person.
Author Contributions
Concept and design: SM and MB. Analysis and interpretation: SM, FM, and GR. Data collection: SM, RR, FM, GR, and UAG. Writing the article: SM. Critical revision of the article: SM, RR, FM, UAG, and MB. Final approval of the article: SM, FM, and MB. Statistical analysis: SM, FM, and GR. Overall responsibility: SM.
Fig. 1.

Fig. 2.
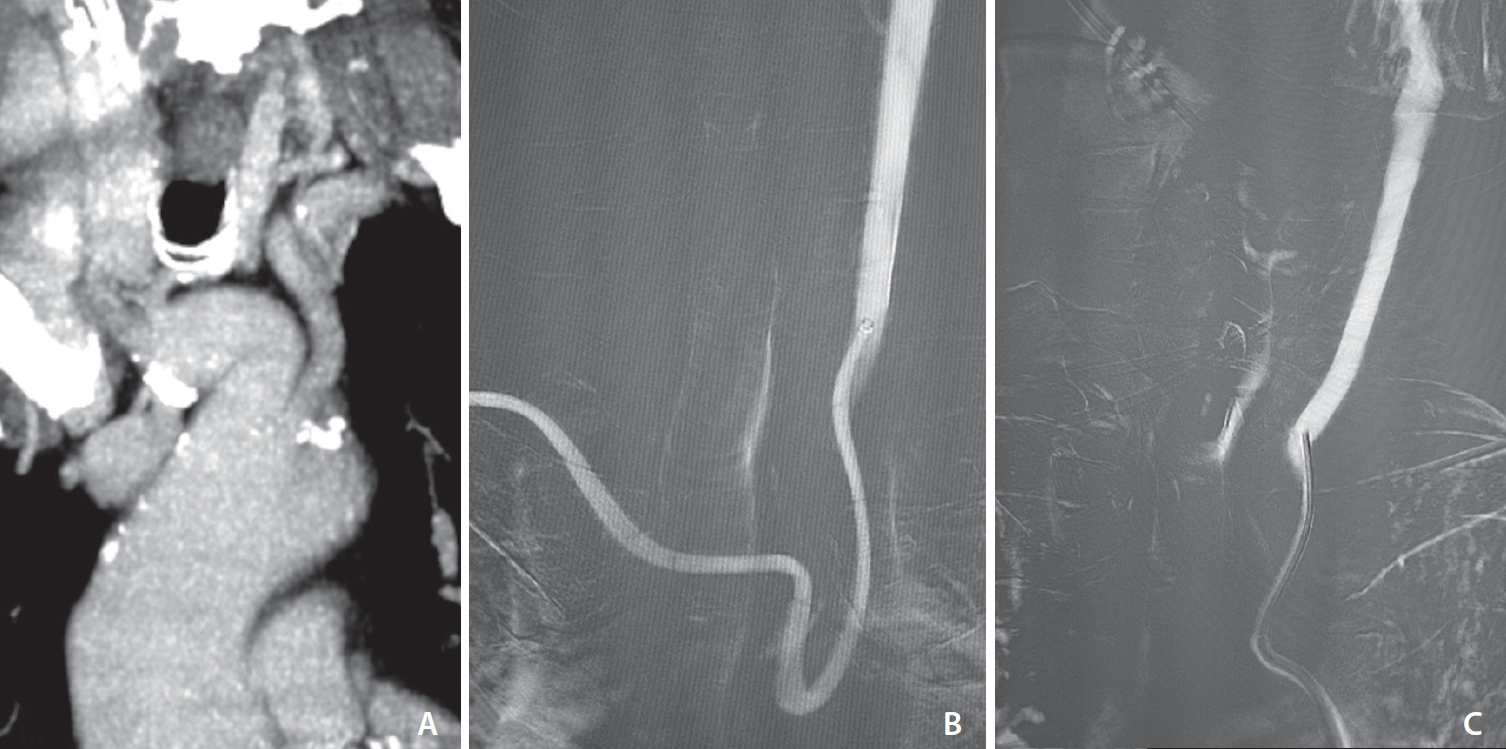
Fig. 3.
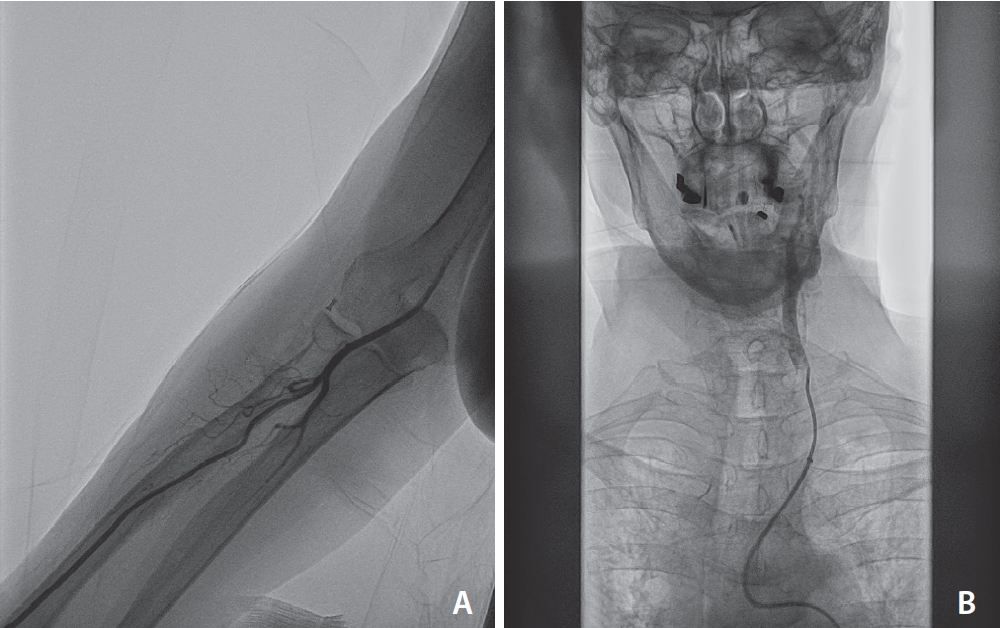
Table 1.
Values are presented as mean±standard deviation (range), mean (range), median (range), or number (%).
NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta stroke program early computed tomography score; IV r-tPA, intravenous recombinant tissue-type plasminogen activator; ICA, internal carotid artery; MCA, middle cerebral artery.
Table 2.
| Overall (n=20) | |
|---|---|
| Treatment data | |
| First-line TRA | 17 (85.0) |
| Bail-out TRA after TFA failure | 3 (15.0) |
| Wrist-to-intracranial access (min) | 7 (4–18) |
| Thromboaspiration only (ADAPT) | 6 (30.0) |
| Stentriever only | 1 (5.0) |
| Combined technique | 13 (65.0) |
| Number of passes | 1.6±0.9 |
| mTICI≥2b | 18 (90.0) |
| mTICI≥2c | 16 (80.0) |
| Post-treatment data | |
| 24 h NIHSS | 8.9±7.9 |
| Discharge NIHSS | 8.65±9.06 |
| Intrahospital mortality | 2 (10.0) |
| Overall ICH | 4 (20.0) |
| RAO* | 2 (10.6) |
| 3-mo mRS 0-2† | 9 (50.0) |
Values are presented as number (%), median (range), or mean±standard deviation.
TRA, transradial approach; TFA, transfemoral approach; ADAPT, a direct aspiration, first pass technique; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; ICH, intracranial hemorrhage; RAO, radial artery occlusion; mRS, modified Rankin scale.







