 |
 |
- Search
| Neurointervention > Volume 19(2); 2024 > Article |
|
Abstract
Purpose
Materials and Methods
Results
Acknowledgments
Notes
Ethics Statement
Approval was obtained from the local Ethical Review Board, Aerztekammer Nordrhein, for this retrospective study (number 66/2024). The study was performed in agreement with institutional guidelines and with the ethical standards according to the latest version of the Declaration of Helsinki. Due to the retrospective design of the study, a separate informed patient consent was not required.
The consent for publication is not required as the submission does not include any images or information that may identify the person.
Author Contributions
Concept and design: FB, MSH, and DG. Analysis and interpretation: FB, MAT, KS, and DG. Data collection: FB, MSH, and DG. Writing the article: FB, MAT, and DG. Critical revision of the article: FB, MAT, KS, HL, MSH, and DG. Final approval of the article: FB, MAT, KS, HL, and DG. Statistical analysis: FB, KS, HL, and DG. Overall responsibility: FB.
Fig.┬Ā1.
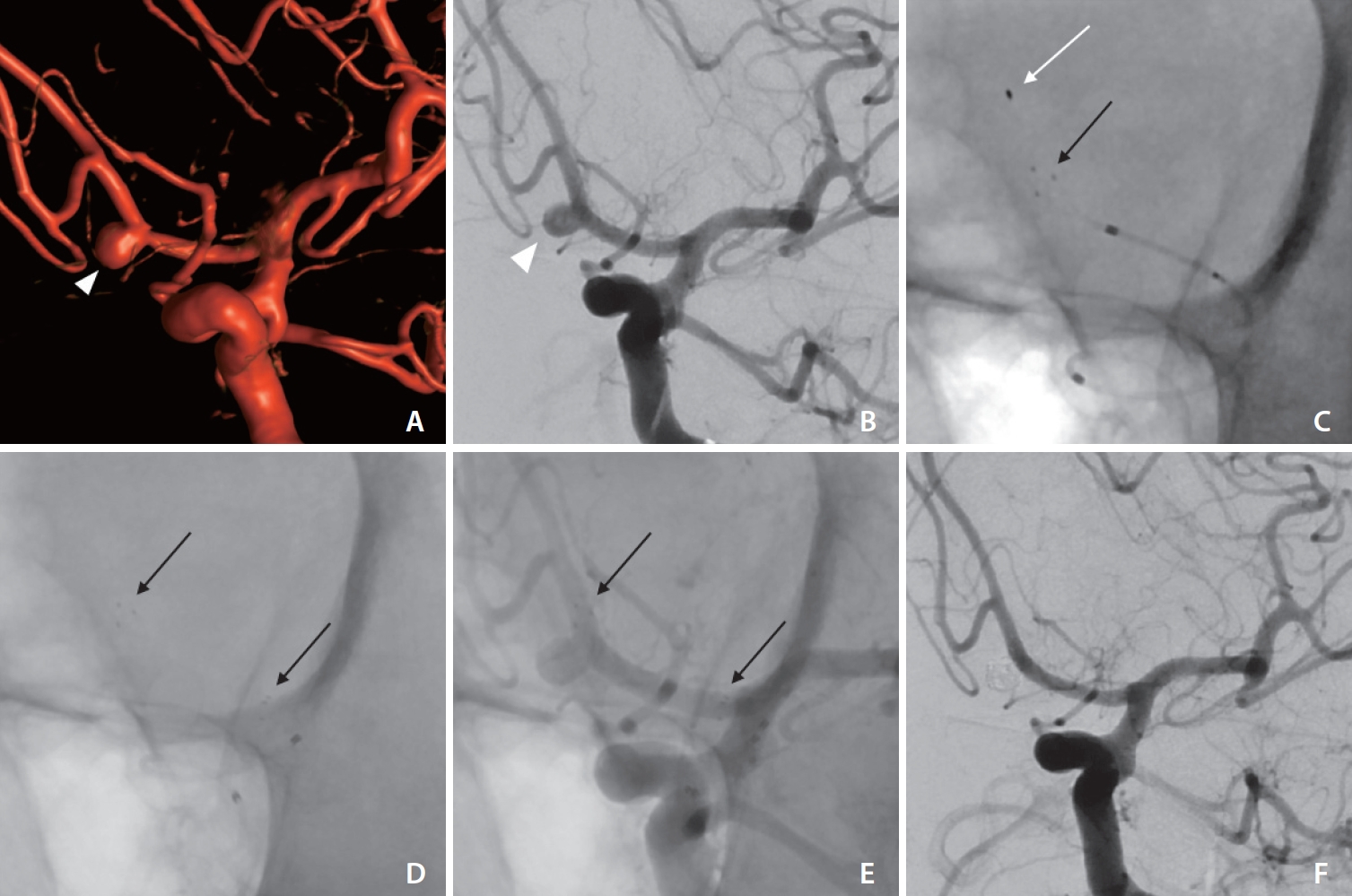
Fig.┬Ā2.
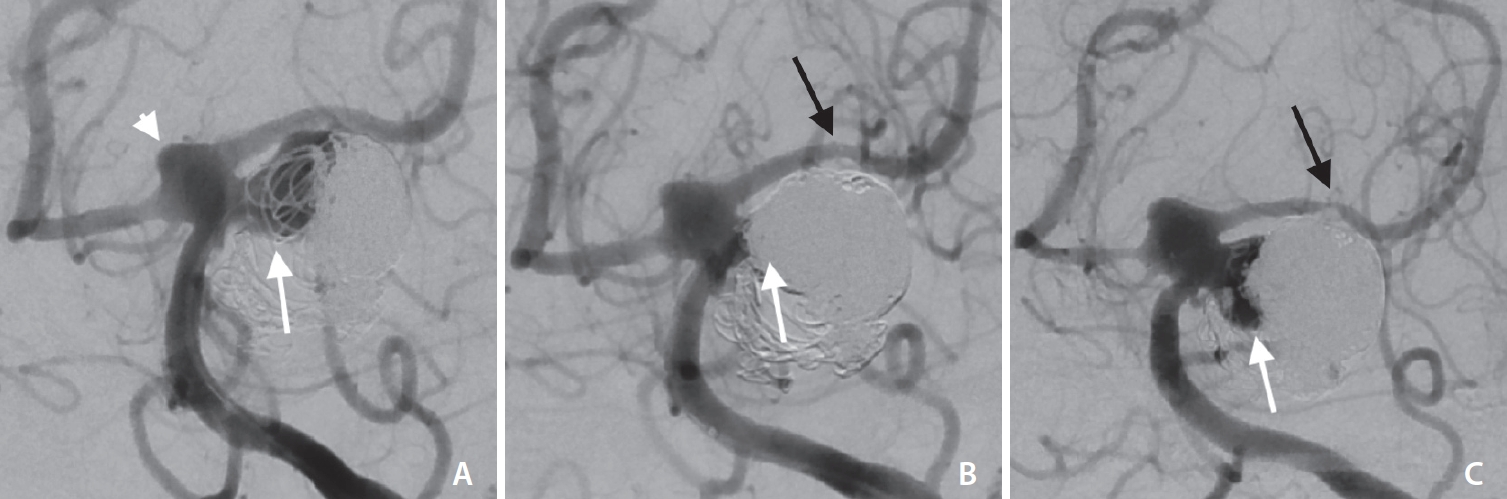
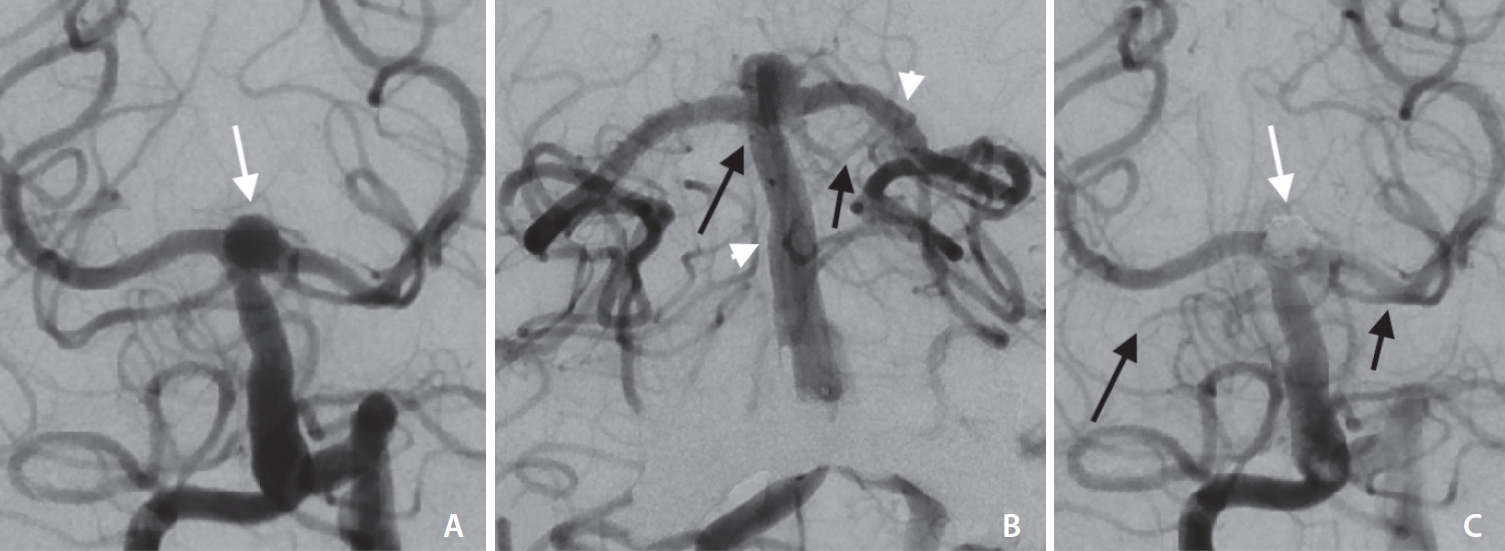
Fig.┬Ā3.
Table┬Ā1.
Values are presented as number (%), number only, or mean┬▒standard deviation.
AcomA, anterior communicating artery; ICA, internal carotid artery; BA, basilar artery; MCA, middle cerebral artery; H, height; W, width; DNR, dome to neck ratio; VD, vessel diameter; SM, stent marker; SAC, stent-assisted coiling; SAH, subarachnoid hemorrhage.
Table┬Ā2.
In all cases, stent-assisted coiling was the treatment of choice except for case 11, where a Woven EndoBridge device was used instead of coilembolization additionally to the pEGASUS stent (Phenox).
Patient 3 died due to complications of the SAH not procedure-related.
VD, vessel diameter in mm with the diameter at the distal (D) and proximal (P) stent marker; RROC, RaymondŌĆōRoy occlusion classification; FU, follow-up; ISS, in-stent stenosis; AcomA, anterior communicating artery; E, elective stent-assisted coiling as initial treatment; BA, basilar artery; SAH, subarachnoid hemorrhage; E (R), elective stent-assisted coiling as retreatment of a recanalized, initially coiled aneurysm; ICA, internal carotid artery; MCA, middle cerebral artery.
REFERENCES
- TOOLS







