 |
 |
- Search
| Neurointervention > Volume 11(1); 2016 > Article |
|
Abstract
Purpose
The purpose of this study was to assess the safety and early outcomes of the Pipeline device for large/giant or fusiform aneurysms.
Materials and Methods
The Pipeline was implanted in a total of 45 patients (mean age, 58 years; M:F=10:35) with 47 large/giant or fusiform aneurysms. We retrospectively evaluated the characteristics of the treated aneurysms, the periprocedural events, morbidity and mortality, and the early outcomes after Pipeline implantation.
Results
The aneurysms were located in the internal carotid artery (ICA) cavernous segment (n=25), ICA intradural segment (n=11), vertebrobasilar trunk (n=8), and middle cerebral artery (n=3). Procedure-related events occurred in 18 cases, consisting of incomplete expansion (n=8), shortening-migration (n=5), transient occlusion of a jailed branch (n=3), and in-stent thrombosis (n=2). Treatment-related morbidity occurred in two patients, but without mortality. Both patients had modified Rankin scale (mRS) scores of 2, but had an improved mRS score of 0 at 1-month follow-up. Of the 19 patients presenting with mass effect, 16 improved but three showed no changes in their presenting symptoms. All patients had excellent outcomes (mRS, 0 or 1) during the follow-up period (median, 6 months; range, 2-30 months). Vascular imaging follow-up (n=31, 65.9%; median, 3 months, range, 1-25 months) showed complete or near occlusion of the aneurysm in 24 patients (77.4%) and decreased sac size in seven patients (22.6%).
Endovascular coiling is currently a standard treatment for intracranial aneurysms. However, coiling still has a higher recurrence rate as compared with clipping, especially in large or giant aneurysms [1]. Strategies to overcome this drawback include the development of various kinds of surface-modified coils or liquid embolic agents [2,3]. However, such attempts have failed or have shown limited eff icacy [1,2,3]. The Pipeline (Covidien, Irvine, CA, USA) is the first stent-like endoluminal device that has shown promising results for the treatment of large/giant, fusiform, or uncoilable complex aneurysms [4,5,6,7,8,9,10]. However, the Pipeline was not launched in Korea until November 2014.
In this study, we report the periprocedural complications, morbidity and mortality, and early clinical and angiographic outcomes after the implantation of the Pipeline for unruptured large/giant (≥15 mm in size) or fusiform aneurysms from an early multicenter experience in Korea.
The Pipeline was implanted in 45 patients with 47 unruptured large/giant or fusiform aneurysms in 12 hospitals in Korea between November 2012 and March 2015. Except for exceptional uses for proctorship purposes, the majority of the Pipeline implants were performed after November 2014, when reimbursement for the Pipeline was allowed. The procedure was conducted with the assistance of the proctors, who had worked in at least 20 Pipeline implantations, until the operator completed at least 5 cases. By the end of the study period, one operator completed ≥10 cases, two operators completed 5-9 cases, seven operators completed 2-4 cases, and five operators completed 1 case.
All procedures were performed under general anesthesia. All patients received dual antiplatelet premedication (aspirin 100-325 mg and clopidogrel 300 mg) for at least f ive days. For all patients, antiplatelet resistance was tested before treatment, and antiplatelet medication was adjusted as recommended by proctors in cases where drug resistance was detected. For large or giant aneurysms with mass effect or with a possible increased risk of delayed rupture, steroid loading was given orally and tapered as previously described [9].
After a 6F shuttle (Cook) or a ≥6F guiding catheter (Envoy, Coddman Neurovascular, CA, USA) was placed in the relevant cervical carotid or vertebral artery, a 0.027-inch Marksman catheter (Covidien, Irvine, CA, USA) was advanced over a 0.014-inch guidewire across the aneurysm neck. In cases where catheter navigation across the aneurysm neck into a parent artery branch distal to the aneurysm was difficult, an exchange technique was applied using a 0.010-inch microcatheter and a 300-cm length exchangeable wire (Transend, Stryker). Next, a Pipeline that matched the largest diameter of the parent artery was introduced and implanted so that it was fully covering the aneurysm neck.
We retrospectively evaluated the presenting symptoms, aneurysm characteristics (type, location, dome and neck sizes, and the presence of an intrasaccular thrombus), the periprocedural events, treatment-related morbidity and mortality, and early clinical and radiological outcomes after Pipeline implantation. Treatment-related morbidity was defined as any neurological deterioration unrelated to preexisting cranial nerve signs due to the aneurysm's mass effect. Clinical outcome was evaluated according to the modified Rankin scale score (mRS) where mRS 0 indicates no symptoms and mRS 6 indicates death. The aneurysm's occlusion at follow-up vascular imaging (catheter angiography or CT angiography) was categorized as complete or near-complete occlusion (aneurysm occlusion > 90%), decreased sac size (30% < aneurysm occlusion < 90%), or no change (aneurysm occlusion < 30%). In addition, the change in the size of the treated aneurysms, including the thrombosed portion, was assessed in cross-sectional follow-up CT or MR images. The institutional review boards of the participating institutions approved this retrospective study and waived patient informed consent based on the study design.
The characteristics of the patients and aneurysms are summarized in Table 1. In two cases of giant intradural aneurysms, several coils were inserted into the sac to more rapidly induce thrombosis. In one case, a stent graft (Jostent graft, Abbott Vascular Devices) was adjunctively used to anchor the Pipeline to the proximal portion of the parent artery because the diameter of the proximal portion was ≥5.5 mm, while the largest Pipeline has a size of 5 mm.
The device-related events and periprocedural complications are summarized in Table 2. Specif ically, incomplete expansion of Pipeline was detected in 13 cases, of which 11 eventually required balloon angioplasty for full expansion of the Pipeline. In-stent thrombosis occurred in two cases, which caused side branch occlusion but completely resolved with intraarterial Glycoprotein IIb/IIIa inhibitor (Tirofiban, 05-1.0 mg) infusion. Another case showed a sluggish flow into the MCA branch but was not treated. The patient had a subclinical infarction in the MCA territory.
Treatment-related morbidity occurred in two patients (4.4%) but without mortality. One patient had a minor embolic infarction in the MCA territory. The other patient had an anterior thalamoperforator infarction due to the retained delivery wire of Pipeline in the posterior communicating artery. Both patients had modified Rankin scale (mRS) scores of 2 after the treatment but had an improved mRS score of 0 at 1-month follow-up.
Of the 18 patients presenting with cranial nerve defects, 15 showed improvements in their presenting symptoms but three patients showed no change. In the 15 patients with improvement, presenting symptoms were initially aggravated 1-3 days posttreatment but improved over 3-8 weeks after Pipeline implantation. The only patient with evidence of brainstem compression also slowly improved in presenting symptoms over three weeks after treatment. All patients had excellent outcomes (mRS, 0 or 1) during the follow-up period with a median of six months (range, 2-30 months).
Vascular imaging follow-up with catheter or CT angiography was available in 31 aneurysms (65.9%) for a median of three months (range, 1-25 months). Vascular imaging follow-up showed complete or near-complete occlusion in 24 patients (77.4%) and decreased sac size in seven patients (22.6%). Asymptomatic in-stent stenosis was detected in one case at the 6-month follow-up angiography, which markedly improved at the 12-month follow-up angiography (Fig. 1). Cross-sectional imaging follow-up at ≥1 month with CT or MR was available in 35 aneurysms (74.5%) for a median of three months (range, 1-25 months). The treated aneurysms, including the thrombosed portions, disappeared in f ive patients (14.3%) (Fig. 2), showed a decreased size in 24 patients (68.6%), and showed no change in six patients (17.1%). In the six cases without changes in the sizes of the thrombosed aneurysm, the latest follow-up cross-sectional images were obtained one to three months after Pipeline implantation. The vascular and cross-sectional imaging results on follow-up are detailed in Table 3.
In the treatment of cerebral aneurysms, coiling is known to have a higher recurrence rate than clipping, especially in large or giant aneurysms [1]. Although a variety of strategies have been attempted to overcome this drawback, they have failed or have shown limited efficacy [1,2,3]. Furthermore, even with stent assistance, coiling is very difficult to perform and especially risky in blister-like, dissecting, or fusiform aneurysms [11,12,13]. The use of single or multiple overlapping stents has helped, to a certain extent, prevent recurrence and treat uncoilable aneurysms [1,11,12,13,14,15,16,17,18,19]. It has also been suggested that overlapping stents could further improve aneurysm healing due to the reinforced flow-diverting effects of increasing the metal density covering the aneurysm neck [16,17,18,19].
The Pipeline, a stent-like endoluminal device with high metal density, was invented for the treatment of aneurysms by flow diversion without coiling, and has been shown to have promising results for the durable occlusion of large or giant aneurysms [4,5,6,7,8,9,10]. After the introduction of the Pipeline, other flow-diverting devices have been invented and applied to the treatment of aneurysms [19,20,21,22]. According to one recent meta-analysis, the complete aneurysmal occlusion rate was 76% overall and 80% for small aneurysms, 74% for large aneurysms, and 76% for giant aneurysms. The procedure-related morbidity and mortality rates were 5% and 4%, respectively. The rate of postoperative subarachnoid hemorrhage was 3% and the rate of intraparenchymal hemorrhage was 3%. The perforator infarction rate was 3%, with significantly lower odds of perforator infarction among patients with aneurysms of the anterior circulation as compared with those of the posterior circulation [23]. Although the risks of procedure-related morbidity and mortality are not negligible, the treatment of intracranial aneurysms with flow-diverters is feasible and effective with high complete occlusion rates, especially considering the fact that flow-diverters have mainly been used for the treatment of aneurysms that are large/giant in size, uncoilable, or have failed prior treatment [23].
In this study, the Pipeline, the f irst flow diverter introduced in Korea, showed an approximately 77% complete or near-complete occlusion rate for large/giant or fusiform aneurysms in this multicenter study, although the median follow-up duration was only three months. In the study duration, there have been no cases of intracranial hemorrhage after Pipeline implantation, either related or unrelated to the treated aneurysms. The treatment-related morbidity was 4.4% (n=2, mRS, 2 in both cases), without any cases of disabling morbidity or mortality. Several factors might have affected these morbidity and mortality rates, which are lower than those reported in the literature. For one, operators could prepare for the known complications of Pipeline implantation by reading previous reports. For example, steroids were given for cases involving giant aneurysms, which have a high risk of delayed aneurysm rupture or an aggravation of the mass effect, as previously described [9]. According to previous reports, the risk of rupture in Pipeline-treated aneurysms increases with aneurysm size [9]. In addition, antiplatelet premedication was modified according to tests for drug resistance, as recommended by the proctors. Finally, in almost all of the cases, the procedure was performed with assistance from experienced proctors. These proctors could decrease treatment-related morbidity by advising operators on how to avoid and manage device-related events. Despite these factors that may have reduced morbidity and mortality, the considerable incidence of device-related events is notable. The most common device-related event was associated with deployment difficulties, such as incomplete expansion and shortening-migration, especially in tortuous parent arteries, which required ballooning post-Pipeline and even additional Pipeline implantations in a few cases (Fig. 1). This is likely because the Pipeline is mainly composed of a Co-Cr alloy strand that has less self-expanding force than nitinol alloy. Another explanation is that the technique for Pipeline implantation is quite different from the technique currently used with neurovascular stents that are made by the laser-cutting of a nitinol tube. The operators tended to have little or no experience in the use of the Pipeline.
This study has several limitations. The number of included cases was relatively small. In addition, follow-up was not completed in all cases and the follow-up period was not long enough. We also did not compare the Pipeline with stent-assisted coiling in the treatment of large or giant aneurysms. Despite these limitations, this report was intended to offer early results from an initial multicenter experience with the Pipeline device. The results of this report can offer future users of flow diverters in Korea with helpful information for improving outcomes. Future studies on flow-diverters should focus on aneurysms that are ≥ 10 mm in size, ruptured blood blister-like or dissecting, recurrent, and uncoilable.
References
1. Crobeddu E, Lanzino G, Kallmes DF, Cloft HJ. Review of 2 decades of aneurysm-recurrence literature, part 1: reducing recurrence after endovascular coiling. AJNR Am J Neuroradiol 2013;34:266-270 .



2. White PM, Raymond J. Endovascular coiling of cerebral aneurysms using "bioactive" or coated-coil technologies: a systematic review of the literature. AJNR Am J Neuroradiol 2009;30:219-226 .



3. Molyneux AJ, Cekirge S, Saatci I, Ga′l G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39-51 .


4. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysm. AJNR Am J Neuroradiol 2009;30:1153-1158 .



5. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009;64:632-642 .



6. Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke 2010;41:2247-2253 .


7. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology 2012;265:893-901 .


8. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858-868 .


9. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012;33:1436-1446 .



10. Chiu AH, Cheung AK, Wenderoth JD, De Villiers L, Rice H, Phatouros CC, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2015;5;[Epub ahead of print]

11. Lee BH, Kim BM, Park MS, Park SI, Chung EC, Suh SH, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg 2009;110:431-436 .


12. Suh SH, Kim BM, Park SI, Kim DI, Shin YS, Kim EJ, et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. J Neurosurg 2009;111:48-52 .


13. Jeon P, Kim BM, Kim DI, Park SI, Kim KH, Kim DJ, et al. Reconstructive endovascular treatment of fusiform or ultrawideneck circumferential aneurysms with multiple overlatpping enterprise stents and coiling. AJNR Am J Neuroradiol 2012;33:965-971 .



14. Piotin M, Blanc R, Sepelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive patients. Stroke 2010;41:110-115 .


15. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013;44:1348-1353 .


16. Kim BM, Kim DJ, Kim DI. Stent application for the treatment of cerebral aneurysms. Neurointervention 2011;6:53-70 .



17. Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 2009;30:1351-1356 .



18. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial veretebrobasilar dissecting aneurysms. Stroke 2011;42:2425-2430 .


19. Lim YC, Kim BM, Suh SH, Jeon P, Kim SH, Ihn YK, et al. Reconsctructive treatment of ruptured blood blister-like aneurysms with stent and coil. Neurosurgery 2013;73:480-488 .



20. Tahtinen OI, Manninen HI, Vanninen RL, Seppanen J, Niskakangas T, Rinne J, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery 2012;70:617-623 .



21. De Vries J, Boogaarts J, Van Norden A, Wakhloo AK. New generation of Low Diverter (surpass) for unruptured intracranial aneurysms: a prospective single-center study in 37 patients. Stroke 2013;44:1567-1577 .


Fig. 1
A 51-year-old woman with bilateral distal internal carotid artery aneurysms.
A, B. Frontal projection and 3-D reconstruction images show a large aneurysm at the intradural para-ophthalmic segment of the right internal carotid artery. The asterisk indicates a coil-embolized aneurysm at the left distal internal carotid artery. The asterisk indicates the coil embolized aneurysm of left internal carotid artery. C. Angiogram after Pipeline implantation shows markedly decreased flow into the aneurysm sac. D. A flat-panel CT image shows incomplete expansion of the distal portion of the Pipeline resulting in poor wall apposition (arrow). E. After ballooning for wall apposition of the Pipeline, the distal end (arrowhead) of the pipeline was partially herniated into the aneurysm sac, resulting in alleviation of the flow diversion effect. F. A spot image after the second Pipeline implantation in a telescopic manner. Note the waist of the second Pipeline at the distal end (arrowhead) of the first Pipeline, which partially herniated into the sac. G. After using a balloon for the apposition of the second Pipeline, the aneurysm sac was no longer visualized. The arrowhead indicates the distal end partially herniated into the sac. H. A flat-panel CT image showing that a mild degree of the waist of the second pipeline still remained at the end of the first pipeline. Note the twisted struts (arrows) at the waist point. I. The 6-month follow-up angiogram shows a severe degree of in-stent stenosis, even though the patient remained asymptomatic. The left anterior cerebral artery was supplied via the anterior communicating artery from the left internal carotid artery (not shown). J. The 12-month follow-up angiogram shows improvement of the in-stent stenosis.
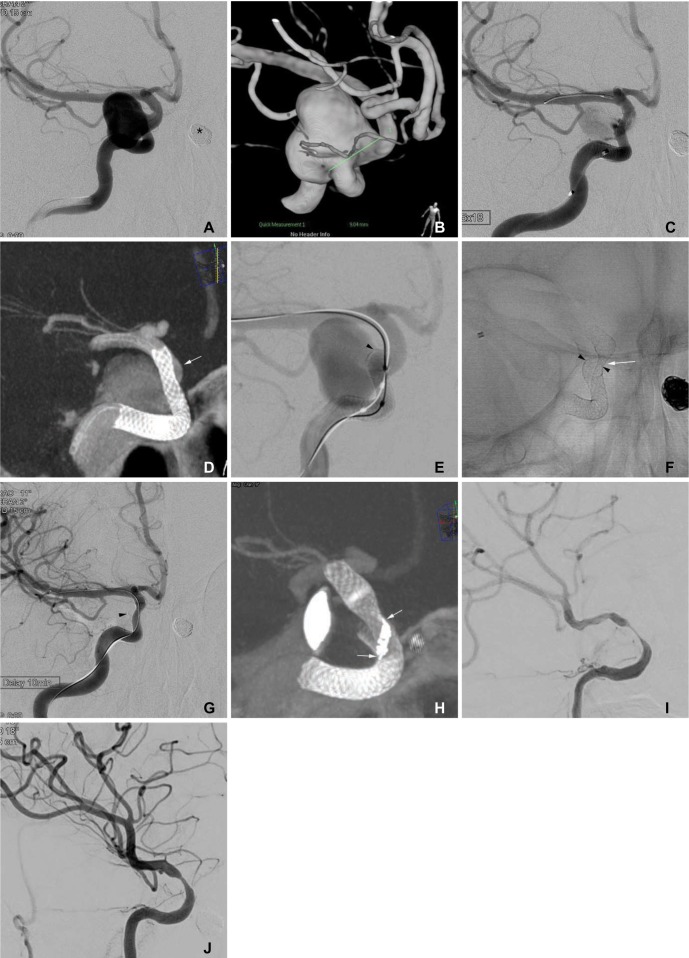
Fig. 2
A 67-year-old woman presenting with third and sixth cranial nerve palsies and a recently aggravated intractable headache.
A. The coronal MIP reconstruction image of the CT angiogram shows a giant aneurysm at the left internal carotid artery, cavernous segment. Note that the left distal internal carotid and middle cerebral arteries were tented (arrow) due to mass effect from the aneurysm. B. A lateral projection angiogram shows a giant aneurysm at the left internal carotid, cavernous segment. C. Final control angiogram after the pipeline implantation shows marked flow diversion with contrast material stagnation in the aneurysm sac. D. A source image of the 3-month follow-up CT angiogram shows that the aneurysm was completely thrombosed. The bulging contour of the left cavernous sinus (arrowheads) remained due to the thrombosed aneurysm. E, F. The source (E) and coronal MIP reconstruction (F) images of the 6-month follow-up CT angiogram show that the thrombosed aneurysm disappeared and that the left internal carotid artery was patent. Note that the left distal internal carotid and middle cerebral arteries were normal-positioned without tenting (arrow).
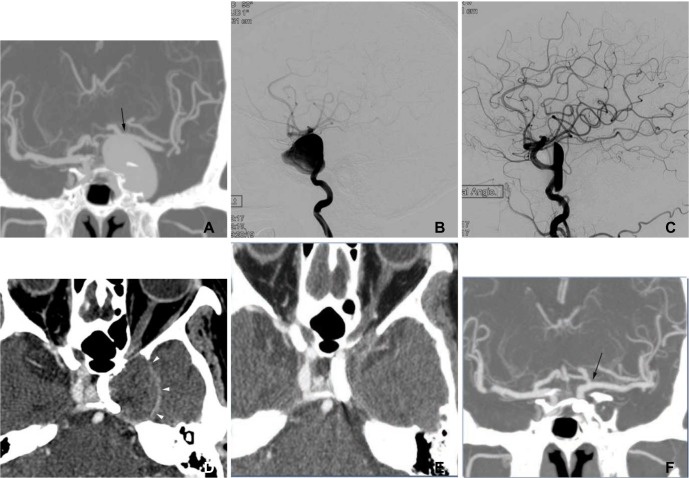
Table 1
The Characteristics of Patients and Aneurysms
Table 2
Procedural Events and Treatment-Related Morbidity
Table 3
Follow-up Imaging Outcomes after Pipeline Implantation
- TOOLS
-
METRICS

-
- 28 Crossref
- 6,727 View
- 122 Download
- Related articles in NI





